CUMYL-4CN-BINACA Is an Efficacious and Potent Pro-Convulsant Synthetic Cannabinoid Receptor Agonist
- PMID: 31191320
- PMCID: PMC6549035
- DOI: 10.3389/fphar.2019.00595
CUMYL-4CN-BINACA Is an Efficacious and Potent Pro-Convulsant Synthetic Cannabinoid Receptor Agonist
Abstract
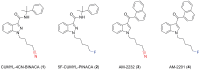
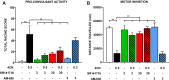
Synthetic cannabinoid receptor agonists (SCRAs) are the largest class of new psychoactive substances (NPS). New examples are detected constantly, and some are associated with a series of adverse effects, including seizures. CUMYL-4CN-BINACA (1-(4-cyanobutyl)-N-(2-phenylpropan-2-yl)indazole-3-carboxamide) is structurally related to potent, cumylamine-derived SCRAs such as 5F-CUMYL-PINACA, but is unusual due to a terminal aliphatic nitrile group not frequently encountered in SCRAs or pharmaceuticals. We report here that CUMYL-4CN-BINACA is a potent CB1 receptor agonist (K i = 2.6 nM; EC50 = 0.58 nM) that produces pro-convulsant effects in mice at a lower dose than reported for any SCRA to date (0.3 mg/kg, i.p). Hypothermic and pro-convulsant effects in mice could be reduced or blocked, respectively, by pretreatment with CB1 receptor antagonist SR141716, pointing to at least partial involvement of CB1 receptors in vivo. Pretreatment with CB2 receptor antagonist AM-630 had no effect on pro-convulsant activity. The pro-convulsant properties and potency of CUMYL-4CN-BINACA may underpin the toxicity associated with this compound in humans.
Keywords: CUMYL; convulsant; new psychoactive substance; novel psychoactive substance; seizure; synthetic cannabinoid.
Figures


Similar articles
-
Synthesis and in Vitro Cannabinoid Receptor 1 Activity of Recently Detected Synthetic Cannabinoids 4F-MDMB-BICA, 5F-MPP-PICA, MMB-4en-PICA, CUMYL-CBMICA, ADB-BINACA, APP-BINACA, 4F-MDMB-BINACA, MDMB-4en-PINACA, A-CHMINACA, 5F-AB-P7AICA, 5F-MDMB-P7AICA, and 5F-AP7AICA.ACS Chem Neurosci. 2020 Dec 16;11(24):4434-4446. doi: 10.1021/acschemneuro.0c00644. Epub 2020 Nov 30. ACS Chem Neurosci. 2020. PMID: 33253529
-
Structural characterization of the new synthetic cannabinoids CUMYL-PINACA, 5F-CUMYL-PINACA, CUMYL-4CN-BINACA, 5F-CUMYL-P7AICA and CUMYL-4CN-B7AICA.Forensic Sci Int. 2017 Dec;281:98-105. doi: 10.1016/j.forsciint.2017.10.020. Epub 2017 Oct 18. Forensic Sci Int. 2017. PMID: 29125990
-
Detection of metabolites of the new synthetic cannabinoid CUMYL-4CN-BINACA in authentic urine samples and human liver microsomes using high-resolution mass spectrometry.Drug Test Anal. 2018 Mar;10(3):449-459. doi: 10.1002/dta.2248. Epub 2017 Aug 24. Drug Test Anal. 2018. PMID: 28691766
-
The Chemistry and Pharmacology of Synthetic Cannabinoid Receptor Agonists as New Psychoactive Substances: Origins.Handb Exp Pharmacol. 2018;252:165-190. doi: 10.1007/164_2018_143. Handb Exp Pharmacol. 2018. PMID: 29980914 Review.
-
Synthetic cannabinoid receptor agonists: classification and nomenclature.Clin Toxicol (Phila). 2020 Feb;58(2):82-98. doi: 10.1080/15563650.2019.1661425. Epub 2019 Sep 16. Clin Toxicol (Phila). 2020. PMID: 31524007 Review.
Cited by
-
Molecular Mechanisms of Action of Novel Psychoactive Substances (NPS). A New Threat for Young Drug Users with Forensic-Toxicological Implications.Life (Basel). 2021 May 14;11(5):440. doi: 10.3390/life11050440. Life (Basel). 2021. PMID: 34068903 Free PMC article. Review.
-
How preclinical studies have influenced novel psychoactive substance legislation in the UK and Europe.Br J Clin Pharmacol. 2020 Mar;86(3):452-481. doi: 10.1111/bcp.14224. Epub 2020 Feb 23. Br J Clin Pharmacol. 2020. PMID: 32045495 Free PMC article. Review.
-
In vitro metabolism of Benzyl-4CN-BUTINACA and MDMB-4CN-BUTINACA using human hepatocytes and LC-QToF-MS analysis.Arch Toxicol. 2025 Jun;99(6):2355-2366. doi: 10.1007/s00204-025-04018-y. Epub 2025 Mar 18. Arch Toxicol. 2025. PMID: 40097708 Free PMC article.
-
Neuronal and peripheral damages induced by synthetic psychoactive substances: an update of recent findings from human and animal studies.Neural Regen Res. 2020 May;15(5):802-816. doi: 10.4103/1673-5374.268895. Neural Regen Res. 2020. PMID: 31719240 Free PMC article. Review.
-
Off-target pharmacological profiling of synthetic cannabinoid receptor agonists including AMB-FUBINACA, CUMYL-PINACA, PB-22, and XLR-11.Front Psychiatry. 2022 Dec 15;13:1048836. doi: 10.3389/fpsyt.2022.1048836. eCollection 2022. Front Psychiatry. 2022. PMID: 36590635 Free PMC article.
References
-
- Abouchedid R., Hudson S., Thurtle N., Yamamoto T., Ho J. H., Bailey G., et al. (2017). Analytical confirmation of synthetic cannabinoids in a cohort of 179 presentations with acute recreational drug toxicity to an Emergency Department in London, UK in the first half of 2015. Clin. Toxicol. 55 (5), 338–345. 10.1080/15563650.2017.1287373 - DOI - PubMed
-
- Åstrand A., Vikingsson S., Lindstedt D., Thelander G., Gréen H., Kronstrand R., et al. (2018). Metabolism study for CUMYL-4CN-BINACA in human hepatocytes and authentic urine specimens: free cyanide is formed during the main metabolic pathway. Drug Test. Anal. 10, 1270–1279. 10.1002/dta.2373 - DOI - PubMed
-
- Arıkan Ölmez N., Kapucu H., Çallı Altun N., Eren B. (2018). Identification of the synthetic cannabinoid N-(2-phenyl-propan-2-yl)-1-(4-cyanobutyl)-1H-indazole-3-carboxamide (CUMYL-4CN-BINACA) in a herbal mixture product. Forensic Toxicol. 36 (1), 192–199. 10.1007/s11419-017-0372-y - DOI
-
- Banister S. D., Longworth M., Kevin R., Sachdev S., Santiago M., Stuart J., et al. (2016). Pharmacology of valinate and tert-leucinate synthetic cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and Their Analogues. ACS Chem. Neurosci. 7 (9), 1241–1254. 10.1021/acschemneuro.6b00137 - DOI - PubMed
LinkOut - more resources
Full Text Sources

