Abnormalities in Glucose Metabolism, Appetite-Related Peptide Release, and Pro- i nflammatory Cytokines Play a Central Role in Appetite Disorders in Peritoneal Dialysis
- PMID: 31191339
- PMCID: PMC6547940
- DOI: 10.3389/fphys.2019.00630
Abnormalities in Glucose Metabolism, Appetite-Related Peptide Release, and Pro- i nflammatory Cytokines Play a Central Role in Appetite Disorders in Peritoneal Dialysis
Abstract
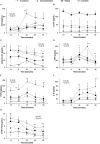
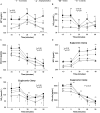
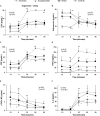
Background: Appetite disorders are frequent and scantly studied in peritoneal dialysis (PD) patients and are associated with malnutrition and cardiovascular complications. Objective: We investigated the relationship between uremic insulin resistance, pro-inflammatory cytokines, and appetite-related peptides release (ARPr) with eating-behavior disorders in PD patients. Methods: We included 42 PD patients (12 suffering anorexia, 12 obese with high food-intake, and 18 asymptomatic) and 10 controls. We measured blood levels of ARPr including orexigens [neuropeptide-Y (NPY), ghrelin, and nitric-oxide], anorexigens [cholecystokinin, insulin, corticotropin-releasing factor, leptin, and adiponectin (Ad)], and cytokines (TNF-α, sTNFα-R2, and IL-6) both at baseline and after administering a standard-food stimulus (SFS). We also measured the expression of TNF-α, leptin and Ad-encoding mRNAs in abdominal adipose tissue. We compared these markers with eating motivation measured by a Visual Analog Scale (VAS). Results: Anorexics showed both little appetite, measured by a VAS, and low levels of orexigens that remained constant after SFS, coupled with high levels of anorexigens at baseline and after SFS. Obeses showed higher appetite, increased baseline levels of orexigens, lower baseline levels of anorexigens and cytokines and two peaks of NPY after SFS. The different patterns of ARPr and cytokines pointed to a close relationship with uremic insulin resistance. In fact, the euglycemic-hyperglycemic clamp reproduced these disorders. In anorexics, TNF-α fat expression was increased. In obese patients, leptin expression in fat tissue was down-regulated and showed correlation with the appetite. Conclusion: In PD, appetite is governed by substances that are altered at baseline and abnormally released. Such modulators are controlled by insulin metabolism and cytokines and, while anorexics display inflammatory predominance, obese patients predominantly display insulin resistance.
Keywords: appetite peptides; euglycemic–hyperglycemic clamp; fat tissue gene expression; insulin resistance; peritoneal dialysis; pro-inflammatory cytokines.
Figures

Similar articles
-
Ghrelin plasma levels and appetite in peritoneal dialysis patients.Adv Perit Dial. 2004;20:194-9. Adv Perit Dial. 2004. PMID: 15384825
-
Eating behavior disorders in uremia: a question of balance in appetite regulation.Semin Dial. 2004 Jan-Feb;17(1):44-52. doi: 10.1046/j.0894-0959.2004.16086.x. Semin Dial. 2004. PMID: 14717811 Review.
-
Anorexigen (TNF-alpha, cholecystokinin) and orexigen (neuropeptide Y) plasma levels in peritoneal dialysis (PD) patients: their relationship with nutritional parameters.Nephrol Dial Transplant. 1998 Jun;13(6):1476-83. doi: 10.1093/ndt/13.6.1476. Nephrol Dial Transplant. 1998. PMID: 9641178
-
Uremic anorexia: a consequence of persistently high brain serotonin levels? The tryptophan/serotonin disorder hypothesis.Perit Dial Int. 2000 Nov-Dec;20(6):810-6. Perit Dial Int. 2000. PMID: 11216590 Review.
-
Cholecystokinin and leptin: their influence upon the eating behaviour and nutrient intake of dialysis patients.Nephrol Dial Transplant. 2004 Jan;19(1):133-40. doi: 10.1093/ndt/gfg471. Nephrol Dial Transplant. 2004. PMID: 14671048
Cited by
-
Updated Role of Neuropeptide Y in Nicotine-Induced Endothelial Dysfunction and Atherosclerosis.Front Cardiovasc Med. 2021 Feb 23;8:630968. doi: 10.3389/fcvm.2021.630968. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 33708805 Free PMC article. Review.
-
Evolving Concepts on Inflammatory Biomarkers and Malnutrition in Chronic Kidney Disease.Nutrients. 2022 Oct 14;14(20):4297. doi: 10.3390/nu14204297. Nutrients. 2022. PMID: 36296981 Free PMC article. Review.
-
Longitudinal Changes of Cytokines and Appetite in Older Hospitalized Patients.Nutrients. 2021 Jul 22;13(8):2508. doi: 10.3390/nu13082508. Nutrients. 2021. PMID: 34444668 Free PMC article.
-
Interaction between central obesity and frailty on the clinical outcome of peritoneal dialysis patients.PLoS One. 2020 Oct 26;15(10):e0241242. doi: 10.1371/journal.pone.0241242. eCollection 2020. PLoS One. 2020. PMID: 33104712 Free PMC article.
References
-
- Adrian J. M., Bloom S. R., Ghatei M. A., Rossor M. N., Roberts G. W., et al. (1983). Neuropeptide Y distribution in human brain. Nature 306 584–586. - PubMed
-
- Aguilera A., Cirugeda A., Amair R., Sansone G., Alegre L., Codoceo R., et al. (2004a). Ghrelin plasma levels and appetite in peritoneal dialysis patients. Adv. Perit. Dial. 20 194–199. - PubMed
-
- Aguilera A., Selgas R., Díez J. J., Bajo M. A., Codoceo R., Alvarez V. (2004b). Eating behavior disorders in uremia: a question of balance in appetite regulation. Semin. Dial. 17 44–52. - PubMed
-
- Aguilera A., Codoceo R., Bajo M. A., Díez J. J., Del Peso G., Pavone M., et al. (2001). Helicobacter pylori infection: a new cause of anorexia in peritoneal dialysis patients. Perit. Dial. Int. 21 S152–S156. - PubMed
-
- Aguilera A., Codoceo R., Bajo M. A., Iglesias P., Diéz J. J., Barril G., et al. (1998). Anorexigen (TNF-α, cholecystokinin) and orexigen (neuropeptide Y) plasma levels in peritoneal dialysis (PD): their relationship with nutritional parameters. Nephrol. Dial. Transplant. 13 1476–1483. - PubMed

