Endotoxin-Induced Emphysema Exacerbation: A Novel Model of Chronic Obstructive Pulmonary Disease Exacerbations Causing Cardiopulmonary Impairment and Diaphragm Dysfunction
- PMID: 31191356
- PMCID: PMC6546905
- DOI: 10.3389/fphys.2019.00664
Endotoxin-Induced Emphysema Exacerbation: A Novel Model of Chronic Obstructive Pulmonary Disease Exacerbations Causing Cardiopulmonary Impairment and Diaphragm Dysfunction
Abstract
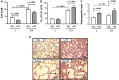
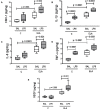
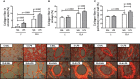
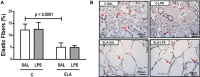
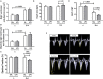
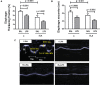
Chronic obstructive pulmonary disease (COPD) is a progressive disorder of the lung parenchyma which also involves extrapulmonary manifestations, such as cardiovascular impairment, diaphragm dysfunction, and frequent exacerbations. The development of animal models is important to elucidate the pathophysiology of COPD exacerbations and enable analysis of possible therapeutic approaches. We aimed to characterize a model of acute emphysema exacerbation and evaluate its consequences on the lung, heart, and diaphragm. Twenty-four Wistar rats were randomly assigned into one of two groups: control (C) or emphysema (ELA). In ELA group, animals received four intratracheal instillations of pancreatic porcine elastase (PPE) at 1-week intervals. The C group received saline under the same protocol. Five weeks after the last instillation, C and ELA animals received saline (SAL) or E. coli lipopolysaccharide (LPS) (200 μg in 200 μl) intratracheally. Twenty-four hours after saline or endotoxin administration, arterial blood gases, lung inflammation and morphometry, collagen fiber content, and lung mechanics were analyzed. Echocardiography, diaphragm ultrasonography (US), and computed tomography (CT) of the chest were done. ELA-LPS animals, compared to ELA-SAL, exhibited decreased arterial oxygenation; increases in alveolar collapse (p < 0.0001), relative neutrophil counts (p = 0.007), levels of cytokine-induced neutrophil chemoattractant-1, interleukin (IL)-1β, tumor necrosis factor-α, IL-6, and vascular endothelial growth factor in lung tissue, collagen fiber deposition in alveolar septa, airways, and pulmonary vessel walls, and dynamic lung elastance (p < 0.0001); reduced pulmonary acceleration time/ejection time ratio, (an indirect index of pulmonary arterial hypertension); decreased diaphragm thickening fraction and excursion; and areas of emphysema associated with heterogeneous alveolar opacities on chest CT. In conclusion, we developed a model of endotoxin-induced emphysema exacerbation that affected not only the lungs but also the heart and diaphragm, thus resembling several features of human disease. This model of emphysema should allow preclinical testing of novel therapies with potential for translation into clinical practice.
Keywords: collagen fiber; diaphragm dysfunction; emphysema; lung mechanics; pulmonary arterial hypertension.
Figures


Similar articles
-
Characterization of a Mouse Model of Emphysema Induced by Multiple Instillations of Low-Dose Elastase.Front Physiol. 2016 Oct 7;7:457. doi: 10.3389/fphys.2016.00457. eCollection 2016. Front Physiol. 2016. PMID: 27774071 Free PMC article.
-
Ghrelin therapy improves lung and cardiovascular function in experimental emphysema.Respir Res. 2017 Nov 3;18(1):185. doi: 10.1186/s12931-017-0668-9. Respir Res. 2017. PMID: 29100513 Free PMC article.
-
Comparison between Variable and Conventional Volume-Controlled Ventilation on Cardiorespiratory Parameters in Experimental Emphysema.Front Physiol. 2016 Jun 30;7:277. doi: 10.3389/fphys.2016.00277. eCollection 2016. Front Physiol. 2016. PMID: 27445862 Free PMC article.
-
Impact of one versus two doses of mesenchymal stromal cells on lung and cardiovascular repair in experimental emphysema.Stem Cell Res Ther. 2018 Nov 8;9(1):296. doi: 10.1186/s13287-018-1043-6. Stem Cell Res Ther. 2018. PMID: 30409216 Free PMC article.
-
The Use of Diaphragm Ultrasonography in Pulmonary Physiotherapy of COPD Patients: A Literature Review.J Clin Med. 2020 Oct 31;9(11):3525. doi: 10.3390/jcm9113525. J Clin Med. 2020. PMID: 33142746 Free PMC article. Review.
Cited by
-
Cause or Effect? Stretching to Understand the Inflammatory Role of Elastin Fiber Breakdown in Chronic Obstructive Pulmonary Disease.Am J Respir Cell Mol Biol. 2020 Nov;63(5):558-559. doi: 10.1165/rcmb.2020-0348ED. Am J Respir Cell Mol Biol. 2020. PMID: 32857600 Free PMC article. No abstract available.
-
The Role of the Extracellular Matrix in the Pathogenesis and Treatment of Pulmonary Emphysema.Int J Mol Sci. 2024 Oct 2;25(19):10613. doi: 10.3390/ijms251910613. Int J Mol Sci. 2024. PMID: 39408941 Free PMC article. Review.
-
CXCR7 Antagonism Reduces Acute Lung Injury Pathogenesis.Front Pharmacol. 2021 Nov 5;12:748740. doi: 10.3389/fphar.2021.748740. eCollection 2021. Front Pharmacol. 2021. PMID: 34803691 Free PMC article.
-
Expression of Matrix Metalloproteinase-2, Matrix Metalloproteinase-9, Tissue Inhibitor of Metalloproteinase-1, and Changes in Alveolar Septa in Patients with Chronic Obstructive Pulmonary Disease.Med Sci Monit. 2020 Oct 18;26:e925278. doi: 10.12659/MSM.925278. Med Sci Monit. 2020. PMID: 33070147 Free PMC article. Clinical Trial.
-
SARS-CoV-2 spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity.J Mol Cell Biol. 2020 Oct 12;12(12):916-932. doi: 10.1093/jmcb/mjaa067. J Mol Cell Biol. 2020. PMID: 33295606 Free PMC article.
References
-
- Abboud R. T., Vimalanathan S. (2008). Pathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysema. Int. J. Tuberc. Lung Dis. 12, 361–367. PMID: - PubMed
LinkOut - more resources
Full Text Sources

