Anti-müllerian Hormone for the Prediction of Ovarian Response in Progestin-Primed Ovarian Stimulation Protocol for IVF
- PMID: 31191453
- PMCID: PMC6547790
- DOI: 10.3389/fendo.2019.00325
Anti-müllerian Hormone for the Prediction of Ovarian Response in Progestin-Primed Ovarian Stimulation Protocol for IVF
Abstract
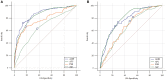
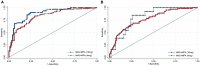
Background: The ability of anti-Müllerian hormone (AMH) to predict ovarian response has been studied extensively in gonadotropin-releasing hormone agonist and antagonist treatments, but no information is available regarding its value in progestin-primed ovarian stimulation (PPOS) protocol. Methods: This retrospective data analysis included 523 patients without polycystic ovary syndrome who underwent their first in vitro fertilization/intracytoplasmic sperm injection cycle with PPOS protocol at our center between Jan. 2015 and Jul. 2018. Serum AMH measurements were acquired within 12 months prior to ovarian stimulation using the automated Access AMH assay. Results: AMH exhibited a significantly positive correlation with the number of retrieved oocytes (r = 0.744, P < 0.001). For the prediction of poor (<4 oocytes) and high (>15 oocytes) response, AMH had an area under the receiver operating characteristic curve (AUC) of 0.861 and 0.773, corresponding with an optimal cutoff point of 1.26 and 4.34 ng/mL, respectively. When stratified according to the dose of medroxyprogesterone acetate (MPA) (4 mg vs. 10 mg per day), AMH retained its similarly high predictive value for poor (AUC = 0.829 and 0.886, respectively) and high response (AUC = 0.770 and 0.814, respectively) in both groups. Amongst the 314 women who received their first frozen embryo transfer (FET) following PPOS protocol, no significant differences were observed on the rates of biochemical pregnancy, clinical pregnancy, implantation, early miscarriage, multiple pregnancy and ectopic pregnancy (all P > 0.05) across AMH quartiles (≤1.43, 1.44-2.55, 2.56-4.35, >4.35 ng/mL). In a multivariable logistic regression model, age was suggested to be the only independent risk factor for clinical pregnancy (P = 0.011). Conclusions: Our data demonstrated that AMH is an adequate predictor of both high and poor ovarian response in PPOS protocol regardless of MPA dose, but it does not associate with pregnancy outcomes in the first FET cycles in a freeze-all strategy.
Keywords: Anti-Müllerian hormone; freeze-all strategy; ovarian response; pregnancy; progestin-primed ovarian stimulation.
Figures
Similar articles
-
Progestin-primed ovarian stimulation protocol with or without letrozole for patients with normal ovarian reserve: a retrospective cohort study.J Clin Pharm Ther. 2022 Apr;47(4):469-476. doi: 10.1111/jcpt.13567. Epub 2021 Nov 18. J Clin Pharm Ther. 2022. PMID: 34796515
-
New application of dydrogesterone as a part of a progestin-primed ovarian stimulation protocol for IVF: a randomized controlled trial including 516 first IVF/ICSI cycles.Hum Reprod. 2018 Feb 1;33(2):229-237. doi: 10.1093/humrep/dex367. Hum Reprod. 2018. PMID: 29300975 Clinical Trial.
-
Analysis of cumulative live birth rate and perinatal outcomes in young patients with low anti-müllerian hormone levels using two ovulation promotion protocols: A cohort study.Front Endocrinol (Lausanne). 2022 Aug 5;13:938500. doi: 10.3389/fendo.2022.938500. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35992097 Free PMC article.
-
Comparison the effects of progestin-primed ovarian stimulation (PPOS) protocol and GnRH-a long protocol in patients with normal ovarian reserve function.Gynecol Endocrinol. 2023 Dec;39(1):2217263. doi: 10.1080/09513590.2023.2217263. Gynecol Endocrinol. 2023. PMID: 37236243 Review.
-
Efficacy of progestin-primed ovarian stimulation in women with polycystic ovary syndrome undergoing in vitro fertilization: a systematic review and meta-analysis.Front Endocrinol (Lausanne). 2023 Sep 19;14:1224858. doi: 10.3389/fendo.2023.1224858. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37795363 Free PMC article.
Cited by
-
Progestin-Primed Ovarian Stimulation with Dydrogesterone versus Medroxyprogesterone Acetate in Women with Polycystic Ovarian Syndrome for in vitro Fertilization: A Retrospective Cohort Study.Drug Des Devel Ther. 2019 Dec 31;13:4461-4470. doi: 10.2147/DDDT.S230129. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 32099323 Free PMC article.
-
Evaluation of gonadotropin-releasing hormone agonist and antagonist protocols on pregnancy outcomes in POSEIDON groups 3 and 4: A randomized controlled trial.Int J Reprod Biomed. 2025 Jun 10;23(3):241-250. doi: 10.18502/ijrm.v23i3.18775. eCollection 2025 Mar. Int J Reprod Biomed. 2025. PMID: 40567909 Free PMC article.
-
Development and Validation of Prediction Model for High Ovarian Response in In Vitro Fertilization-Embryo Transfer: A Longitudinal Study.Comput Math Methods Med. 2021 Oct 16;2021:7822119. doi: 10.1155/2021/7822119. eCollection 2021. Comput Math Methods Med. 2021. PMID: 34697556 Free PMC article.
-
Ovarian response to controlled stimulation and its predictors in a limited-resource setting.BMC Womens Health. 2024 May 7;24(1):279. doi: 10.1186/s12905-024-02991-7. BMC Womens Health. 2024. PMID: 38714986 Free PMC article.
-
The Value of Anti-Müllerian Hormone in the Prediction of Spontaneous Pregnancy: A Systematic Review and Meta-Analysis.Front Endocrinol (Lausanne). 2021 Oct 13;12:695157. doi: 10.3389/fendo.2021.695157. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34721287 Free PMC article.
References
LinkOut - more resources
Full Text Sources

