Nucleoside Analogues as Antibacterial Agents
- PMID: 31191461
- PMCID: PMC6540614
- DOI: 10.3389/fmicb.2019.00952
Nucleoside Analogues as Antibacterial Agents
Abstract
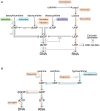
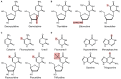
The rapid increase in antibiotic-resistant bacteria has emphasized the urgent need to identify new treatments for bacterial infections. One attractive approach, reducing the need for expensive and time-consuming clinical trials, is to repurpose existing clinically approved compounds for use as antibacterial agents. Nucleoside analogues are commonly used for treating viral and fungal infections, as well as for treating cancers, but have received relatively little attention as treatments for bacterial infections. However, a significant number of clinically approved derivatives of both pyrimidines and purines including halogenated, thiolated, and azolated compounds have been shown to have antibacterial activity. In the small number of studies carried out to date, such compounds have shown promise in treating bacterial infections. Here, we review the mechanisms of action and antibacterial activities of nucleoside analogues that can potentially be repurposed for treating infections as well as considering possible limitations in their usage.
Keywords: antibacterial agents; antibiotic resistance; antimicrobial; multidrug-resistant bacteria; purine analogues; pyrimidine analogues; repurposed antibiotics.
Figures
References
-
- Boston Interhospital Virus Study Group and NIAID-Sponsored Cooperative Antiviral Clinical Study (1975). Failure of high dose 5-iodo-2′-deoxyuridine in the therapy of herpes simplex virus encephalitis. Evidence of unacceptable toxicity. N. Engl. J. Med. 292, 599–603. 10.1056/NEJM197503202921201, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

