Higher Bacterial Diversity of Gut Microbiota in Different Natural Populations of Leafhopper Vector Does Not Influence WDV Transmission
- PMID: 31191481
- PMCID: PMC6548887
- DOI: 10.3389/fmicb.2019.01144
Higher Bacterial Diversity of Gut Microbiota in Different Natural Populations of Leafhopper Vector Does Not Influence WDV Transmission
Abstract
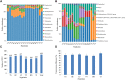
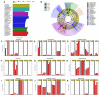
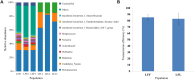
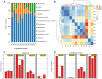
The bacterial communities in the gut of an insect have important ecological and functional effects on the insect. However, the community composition and diversity of the gut microbiota in insects that vector plant viruses are poorly understood. As an important insect vector, Psammotettix alienus transmits various viruses including wheat dwarf virus (WDV). Here, we used the combination of leafhopper and WDV as model to survey the influence of gut microbiota on virus transmission characteristic of insect vector and vice versa. We have characterized 22 phyla and 249 genera of all gut bacterial communities in the leafhopper populations collected from six geographic regions in China. Community composition and diversity varied across different geographic populations. However, WDV transmission efficiencies of these six field populations were all greater than 80% with no significant difference. Interestingly, the transmission efficiency of WDV by laboratory reared insects with decreased gut bacterial diversity was similar to that of field populations. Furthermore, we found that the composition of the leafhopper gut bacteria was dynamic and could reversibly respond to WDV acquisition. Higher bacterial diversity and abundance of gut microbiota in different leafhopper populations did not influence their WDV transmission efficiency, while the acquisition of WDV changes gut microbiota by a dynamic and reversible manner. This report provides insight into the complex relationship between the gut microbiota, insect vector and virus.
Keywords: 16S rDNA high-throughput sequencing; European grass feeding leafhopper (Psammotettix alienus); geographic location; gut bacterial community; wheat dwarf virus (WDV).
Figures

Similar articles
-
ADP ribosylation factor 1 facilitates spread of wheat dwarf virus in its insect vector.Cell Microbiol. 2019 Sep;21(9):e13047. doi: 10.1111/cmi.13047. Epub 2019 Jun 2. Cell Microbiol. 2019. PMID: 31099153
-
First Report of Wheat dwarf virus and Its Vector (Psammotettix provincialis) Affecting Wheat and Barley Crops in Syria.Plant Dis. 2011 Jan;95(1):76. doi: 10.1094/PDIS-09-10-0628. Plant Dis. 2011. PMID: 30743673
-
Localization and distribution of wheat dwarf virus in its vector leafhopper, Psammotettix alienus.Phytopathology. 2014 Aug;104(8):897-904. doi: 10.1094/PHYTO-09-13-0251-R. Phytopathology. 2014. PMID: 24502202
-
The Past, Present, and Future of Wheat Dwarf Virus Management-A Review.Plants (Basel). 2023 Oct 20;12(20):3633. doi: 10.3390/plants12203633. Plants (Basel). 2023. PMID: 37896096 Free PMC article. Review.
-
Beyond 16S rRNA Community Profiling: Intra-Species Diversity in the Gut Microbiota.Front Microbiol. 2016 Sep 21;7:1475. doi: 10.3389/fmicb.2016.01475. eCollection 2016. Front Microbiol. 2016. PMID: 27708630 Free PMC article. Review.
Cited by
-
Roles of Bacterial Symbionts in Transmission of Plant Virus by Hemipteran Vectors.Front Microbiol. 2022 Jan 27;13:805352. doi: 10.3389/fmicb.2022.805352. eCollection 2022. Front Microbiol. 2022. PMID: 35154053 Free PMC article. Review.
-
Gut microorganisms of Locusta migratoria in various life stages and its possible influence on cellulose digestibility.mSystems. 2024 Jul 23;9(7):e0060024. doi: 10.1128/msystems.00600-24. Epub 2024 Jun 18. mSystems. 2024. PMID: 38888356 Free PMC article.
-
Rice black-streaked dwarf virus: From multiparty interactions among plant-virus-vector to intermittent epidemics.Mol Plant Pathol. 2020 Aug;21(8):1007-1019. doi: 10.1111/mpp.12946. Epub 2020 Jun 8. Mol Plant Pathol. 2020. PMID: 32510844 Free PMC article.
-
Parasitism by endoparasitoid wasps alters the internal but not the external microbiome in host caterpillars.Anim Microbiome. 2021 Oct 15;3(1):73. doi: 10.1186/s42523-021-00135-y. Anim Microbiome. 2021. PMID: 34654483 Free PMC article.
-
Caterpillar-parasitoid interactions: species-specific influences on host microbiome composition.FEMS Microbiol Ecol. 2024 Sep 14;100(10):fiae115. doi: 10.1093/femsec/fiae115. FEMS Microbiol Ecol. 2024. PMID: 39165109 Free PMC article.
References
-
- Ben Guerrero E., Soria M., Salvador R., Ceja-Navarro J. A., Campos E., Brodie E. L., et al. (2016). Effect of different lignocellulosic diets on bacterial microbiota and hydrolytic enzyme activities in the gut of the cotton boll weevil (Anthonomus grandis). Front. Microbiol. 7:2093. 10.3389/fmicb.2016.02093 - DOI - PMC - PubMed
-
- Benkovics A. H., Vida G., Nelson D., Veisz O., Bedford I., Silhavy D., et al. (2010). Partial resistance to wheat dwarf virus in winter wheat cultivars. Plant Pathol. 59 1144–1151. 10.1111/j.1365-3059.2010.02318.x - DOI
LinkOut - more resources
Full Text Sources
Research Materials

