Seneca Valley Virus 2C and 3Cpro Induce Apoptosis via Mitochondrion-Mediated Intrinsic Pathway
- PMID: 31191506
- PMCID: PMC6549803
- DOI: 10.3389/fmicb.2019.01202
Seneca Valley Virus 2C and 3Cpro Induce Apoptosis via Mitochondrion-Mediated Intrinsic Pathway
Abstract
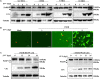
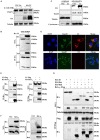
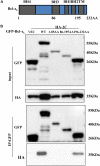
Seneca Valley virus (SVV) is the only member of the genus Senecavirus of the Picornaviridae family. SVV can selectively infect and lyse tumor cells with neuroendocrine features and is used as an oncolytic virus for treating small-cell lung cancers. However, the detailed mechanism underlying SVV-mediated destruction of tumor cells remains unclear. In this study, we found that SVV can increase the proportion of apoptotic 293T cells in a dose- and time-dependent manner. SVV-induced apoptosis was initiated via extrinsic and intrinsic pathways through activation of caspase-3, the activity of which could be attenuated by a pan-caspase inhibitor (Z-VAD-FMK). We confirmed that SVV 2C and 3Cpro play critical roles in SVV-induced apoptosis. The SVV 2C protein was located solely in the mitochondria and activated caspase-3 to induce apoptosis. SVV 3Cpro induced apoptosis through its protease activity, which was accompanied by release of cytochrome C into the cytoplasm, but did not directly cleave PARP1.
Keywords: 2C; 3Cpro; apoptosis; caspase-3; seneca valley virus (SVV).
Figures



Similar articles
-
Seneca Valley virus 3Cpro antagonizes host innate immune responses and programmed cell death.Front Microbiol. 2023 Oct 6;14:1235620. doi: 10.3389/fmicb.2023.1235620. eCollection 2023. Front Microbiol. 2023. PMID: 37869659 Free PMC article. Review.
-
Seneca Valley Virus 3Cpro Cleaves PABPC1 to Promote Viral Replication.Pathogens. 2020 Jun 4;9(6):443. doi: 10.3390/pathogens9060443. Pathogens. 2020. PMID: 32512928 Free PMC article.
-
Seneca Valley virus 3C protease cleaves HDAC4 to antagonize type I interferon signaling.J Virol. 2025 Mar 18;99(3):e0217624. doi: 10.1128/jvi.02176-24. Epub 2025 Feb 10. J Virol. 2025. PMID: 39927774 Free PMC article.
-
Seneca Valley virus 2C and 3C inhibit type I interferon production by inducing the degradation of RIG-I.Virology. 2019 Sep;535:122-129. doi: 10.1016/j.virol.2019.06.017. Epub 2019 Jun 28. Virology. 2019. PMID: 31299488
-
Oncolytic Seneca Valley Virus: past perspectives and future directions.Oncolytic Virother. 2016 Sep 6;5:81-9. doi: 10.2147/OV.S96915. eCollection 2016. Oncolytic Virother. 2016. PMID: 27660749 Free PMC article. Review.
Cited by
-
RSAD2 suppresses viral replication by interacting with the Senecavirus A 2 C protein.Vet Res. 2024 Sep 27;55(1):115. doi: 10.1186/s13567-024-01370-2. Vet Res. 2024. PMID: 39334325 Free PMC article.
-
Picornavirus 2C proteins: structure-function relationships and interactions with host factors.Front Cell Infect Microbiol. 2024 Feb 23;14:1347615. doi: 10.3389/fcimb.2024.1347615. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38465233 Free PMC article. Review.
-
Regulation of Apoptosis by Enteroviruses.Front Microbiol. 2020 Jun 3;11:1145. doi: 10.3389/fmicb.2020.01145. eCollection 2020. Front Microbiol. 2020. PMID: 32582091 Free PMC article. Review.
-
Seneca Valley virus 3Cpro antagonizes host innate immune responses and programmed cell death.Front Microbiol. 2023 Oct 6;14:1235620. doi: 10.3389/fmicb.2023.1235620. eCollection 2023. Front Microbiol. 2023. PMID: 37869659 Free PMC article. Review.
-
Senecavirus A induces mitophagy to promote self-replication through direct interaction of 2C protein with K27-linked ubiquitinated TUFM catalyzed by RNF185.Autophagy. 2024 Jun;20(6):1286-1313. doi: 10.1080/15548627.2023.2293442. Epub 2024 Jan 3. Autophagy. 2024. PMID: 38084826 Free PMC article.
References
-
- Acehan D., Jiang X., Morgan D. G., Heuser J. E., Wang X., Akey C. W. (2002). Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol. Cell 9 423–432. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

