Inflammatory Phenotype of Intrahepatic Sulfatide-Reactive Type II NKT Cells in Humans With Autoimmune Hepatitis
- PMID: 31191516
- PMCID: PMC6546815
- DOI: 10.3389/fimmu.2019.01065
Inflammatory Phenotype of Intrahepatic Sulfatide-Reactive Type II NKT Cells in Humans With Autoimmune Hepatitis
Abstract
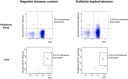
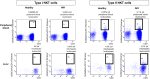
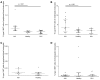
Background: Natural Killer T (NKT) cells are CD1d-restricted innate-like T cells that can rapidly release stored cytokines upon recognition of lipid antigens. In mice, type I NKT cells seem to promote liver inflammation, whereas type II NKT cells seem to restrict hepatitis. Here, we aimed at characterizing the role of human type I and type II NKT in patients with autoimmune hepatitis (AIH). Methods: NKT cells were analyzed by flow cytometry in peripheral blood and liver of AIH patients and control groups. α-galactosylceramide-loaded or sulfatide-loaded tetramers were used to detect type I or II NKT cells, respectively. Hepatic CD1d was stained by in situ-hybridization of liver biopsies. Results and Conclusions: Type II NKT cells were more prevalent in human peripheral blood and liver than type I NKT cells. In AIH patients, the frequency of sulfatide-reactive type II NKT cells was significantly increased in peripheral blood (0.11% of peripheral blood leukocytes) and liver (3.78% of intrahepatic leukocytes) compared to healthy individuals (0.05% and 1.82%) and patients with drug-induced liver injury (0.06% and 2.03%; p < 0.05). Intrahepatic type II NKT cells of AIH patients had a different cytokine profile than healthy subjects with an increased frequency of TNFα (77.8% vs. 59.1%, p < 0.05), decreased IFNγ (32.7% vs. 63.0%, p < 0.05) and a complete lack of IL-4 expressing cells (0% vs. 2.1%, p < 0.05). T cells in portal tracts expressed significantly more CD1d-RNA in AIH livers compared to controls. This study supports that in contrast to their assumed protective role in mice, human intrahepatic, sulfatide-reactive type II NKT cells displayed a proinflammatory cytokine profile in patients with AIH. Infiltrating T cells in portal areas of AIH patients overexpressed CD1d and could thereby activate type II NKT cells.
Keywords: CD1d; alpha-galactosylceramide; lipid antigen; tumor necrosis factor-alpha; unconventional T cells.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

