IL-1β-Mediated Activation of Adipose-Derived Mesenchymal Stromal Cells Results in PMN Reallocation and Enhanced Phagocytosis: A Possible Mechanism for the Reduction of Osteoarthritis Pathology
- PMID: 31191517
- PMCID: PMC6545928
- DOI: 10.3389/fimmu.2019.01075
IL-1β-Mediated Activation of Adipose-Derived Mesenchymal Stromal Cells Results in PMN Reallocation and Enhanced Phagocytosis: A Possible Mechanism for the Reduction of Osteoarthritis Pathology
Abstract
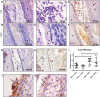
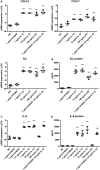
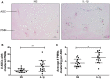
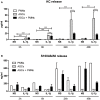
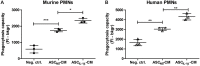
Background: Injection of adipose-derived mesenchymal stromal cells (ASCs) into murine knee joints after induction of inflammatory collagenase-induced osteoarthritis (CiOA) reduces development of joint pathology. This protection is only achieved when ASCs are applied in early CiOA, which is characterized by synovitis and high S100A8/A9 and IL-1β levels, suggesting that inflammation is a prerequisite for the protective effect of ASCs. Our objective was to gain more insight into the interplay between synovitis and ASC-mediated amelioration of CiOA pathology. Methods: CiOA was induced by intra-articular collagenase injection. Knee joint sections were stained with hematoxylin/eosin and immunolocalization of polymorphonuclear cells (PMNs) and ASCs was performed using antibodies for NIMP-R14 and CD271, respectively. Chemokine expression induced by IL-1β or S100A8/A9 was assessed with qPCR and Luminex. ASC-PMN co-cultures were analyzed microscopically and with Luminex for inflammatory mediators. Migration of PMNs through transwell membranes toward conditioned medium of non-stimulated ASCs (ASCNS-CM) or IL-1β-stimulated ASCs (ASCIL-1β-CM) was examined using flow cytometry. Phagocytic capacity of PMNs was measured with labeled zymosan particles. Results: Intra-articular saline injection on day 7 of CiOA increased synovitis after 6 h, characterized by PMNs scattered throughout the joint cavity and the synovium. ASC injection resulted in comparable numbers of PMNs which clustered around ASCs in close interaction with the synovial lining. IL-1β-stimulation of ASCs in vitro strongly increased expression of PMN-attracting chemokines CXCL5, CXCL7, and KC, whereas S100A8/A9-stimulation did not. In agreement, the number of clustered PMNs per ASC was significantly increased after 6 h of co-culturing with IL-1β-stimulated ASCs. Also migration of PMNs toward ASCIL-1β-CM was significantly enhanced (287%) when compared to ASCNS-CM. Interestingly, association of PMNs with ASCs significantly diminished KC protein release by ASCs (69% lower after 24 h), accompanied by reduced release of S100A8/A9 protein by the PMNs. Moreover, phagocytic capacity of PMNs was strongly enhanced after priming with ASCIL-1β-CM. Conclusions: Local application of ASCs in inflamed CiOA knee joints results in clustering of attracted PMNs with ASCs in the synovium, which is likely mediated by IL-1β-induced up-regulation of chemokine release by ASCs. This results in enhanced phagocytic capacity of PMNs, enabling the clearance of debris to attenuate synovitis.
Keywords: CiOA; PMNs; adipose-derived mesenchymal stromal cells; chemokines; interleukin-1β; phagocytosis; synovitis.
Figures

Similar articles
-
Treatment efficacy of adipose-derived stem cells in experimental osteoarthritis is driven by high synovial activation and reflected by S100A8/A9 serum levels.Osteoarthritis Cartilage. 2014 Aug;22(8):1158-66. doi: 10.1016/j.joca.2014.05.022. Epub 2014 Jun 10. Osteoarthritis Cartilage. 2014. PMID: 24928317
-
The role of NOX2-derived reactive oxygen species in collagenase-induced osteoarthritis.Osteoarthritis Cartilage. 2018 Dec;26(12):1722-1732. doi: 10.1016/j.joca.2018.08.014. Epub 2018 Sep 5. Osteoarthritis Cartilage. 2018. PMID: 30195046
-
S100A8/A9 increases the mobilization of pro-inflammatory Ly6Chigh monocytes to the synovium during experimental osteoarthritis.Arthritis Res Ther. 2017 Sep 29;19(1):217. doi: 10.1186/s13075-017-1426-6. Arthritis Res Ther. 2017. PMID: 28969686 Free PMC article.
-
The influence of radiographic contrast media on some granulocyte functions.Acta Radiol Suppl. 1998;419:7-35. Acta Radiol Suppl. 1998. PMID: 9779014 Review.
-
Arf6 regulates energy metabolism in neutrophils.Free Radic Biol Med. 2021 Aug 20;172:550-561. doi: 10.1016/j.freeradbiomed.2021.07.001. Epub 2021 Jul 7. Free Radic Biol Med. 2021. PMID: 34245858 Review.
Cited by
-
Cell-based therapies have disease-modifying effects on osteoarthritis in animal models. A systematic review by the ESSKA Orthobiologic Initiative. Part 1: adipose tissue-derived cell-based injectable therapies.Knee Surg Sports Traumatol Arthrosc. 2023 Feb;31(2):641-655. doi: 10.1007/s00167-022-07063-7. Epub 2022 Sep 14. Knee Surg Sports Traumatol Arthrosc. 2023. PMID: 36104484 Free PMC article.
-
Mesenchymal stem cells in radiation-induced lung injury: From mechanisms to therapeutic potential.Front Cell Dev Biol. 2022 Dec 12;10:1100305. doi: 10.3389/fcell.2022.1100305. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36578783 Free PMC article. Review.
-
Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis.Stem Cell Res Ther. 2022 Jan 10;13(1):14. doi: 10.1186/s13287-021-02689-9. Stem Cell Res Ther. 2022. PMID: 35012666 Free PMC article. Review.
-
Inflammation and Starvation Affect Housekeeping Gene Stability in Adipose Mesenchymal Stromal Cells.Curr Issues Mol Biol. 2024 Jan 19;46(1):842-855. doi: 10.3390/cimb46010054. Curr Issues Mol Biol. 2024. PMID: 38275668 Free PMC article.
-
Exploring Anti-Fibrotic Effects of Adipose-Derived Stem Cells: Transcriptome Analysis upon Fibrotic, Inflammatory, and Hypoxic Conditioning.Cells. 2024 Apr 17;13(8):693. doi: 10.3390/cells13080693. Cells. 2024. PMID: 38667308 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

