Bacterial Protein Toll-Like-Receptor Agonists: A Novel Perspective on Vaccine Adjuvants
- PMID: 31191528
- PMCID: PMC6549121
- DOI: 10.3389/fimmu.2019.01144
Bacterial Protein Toll-Like-Receptor Agonists: A Novel Perspective on Vaccine Adjuvants
Abstract
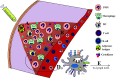
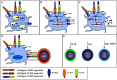
Adjuvants have been used in vaccines for over a century, however, the search for safe and effective vaccine adjuvants continues. In recent decades toll-like-receptor (TLR) agonists have been investigated as potential vaccine adjuvants. In this regard, the majority of the currently investigated TLR agonists are non-protein microbial components such as lipopolysaccharides, oligonucleotides, and lipopeptides. On the other hand, a growing number of studies reveal that TLR signaling and immune responses can be activated by numerous bacterial proteins. However, their potential roles as adjuvants have been somewhat overlooked. Herein, we discuss several such bacterial proteins which exhibit adjuvant properties, including the activation of TLR signaling, antigen presenting cell maturation, pro-inflammatory cytokine production and adaptive immune response. The protein nature of these TLR agonists presents several unique features not shared by non-protein TLR agonists. These properties include the amenability for modifying the structure and function as necessary for optimal immunogenicity and minimal toxicity. Protein adjuvants can be genetically fused to protein antigens which ensure the co-delivery of adjuvant-antigen not only into the same cell but also in the same endocytic cargo, leading to more effective activation of innate and adaptive immune response.
Keywords: TLR; TLR agonist; adjuvant; antigen presenting cells; cell-mediated immunity; vaccine.
Figures
Similar articles
-
Engagement of TLR signaling as adjuvant: towards smarter vaccine and beyond.Vaccine. 2008 Dec 9;26(52):6777-83. doi: 10.1016/j.vaccine.2008.09.045. Epub 2008 Oct 7. Vaccine. 2008. PMID: 18835576 Review.
-
Avian influenza virus vaccines containing Toll-like receptors 2 and 5 ligand adjuvants promote protective immune responses in chickens.Viral Immunol. 2014 May;27(4):160-6. doi: 10.1089/vim.2013.0129. Epub 2014 May 5. Viral Immunol. 2014. PMID: 24797722
-
Activated human neonatal CD8+ T cells are subject to immunomodulation by direct TLR2 or TLR5 stimulation.J Immunol. 2009 Jan 1;182(1):55-62. doi: 10.4049/jimmunol.182.1.55. J Immunol. 2009. PMID: 19109135
-
Co-encapsulation of synthetic lipidated TLR4 and TLR7/8 agonists in the liposomal bilayer results in a rapid, synergistic enhancement of vaccine-mediated humoral immunity.J Control Release. 2019 Dec 10;315:186-196. doi: 10.1016/j.jconrel.2019.10.025. Epub 2019 Oct 22. J Control Release. 2019. PMID: 31654684 Free PMC article.
-
Toll-like receptors as adjuvant receptors.Biochim Biophys Acta. 2002 Feb 13;1589(1):1-13. doi: 10.1016/s0167-4889(01)00182-3. Biochim Biophys Acta. 2002. PMID: 11909637 Review.
Cited by
-
In silico designing of novel epitope-based peptide vaccines against HIV-1.Biotechnol Lett. 2024 Jun;46(3):315-354. doi: 10.1007/s10529-023-03464-x. Epub 2024 Feb 26. Biotechnol Lett. 2024. PMID: 38403788
-
Toll-like receptor 4 signaling-mediated responses are critically engaged in optimal host protection against highly virulent Mycobacterium tuberculosis K infection.Virulence. 2020 Dec;11(1):430-445. doi: 10.1080/21505594.2020.1766401. Virulence. 2020. PMID: 32403973 Free PMC article.
-
Recent Insights Into the Molecular Mechanism of Toll-Like Receptor Response to Dengue Virus Infection.Front Microbiol. 2021 Sep 16;12:744233. doi: 10.3389/fmicb.2021.744233. eCollection 2021. Front Microbiol. 2021. PMID: 34603272 Free PMC article. Review.
-
Is the oral microbiome a source to enhance mucosal immunity against infectious diseases?NPJ Vaccines. 2021 Jun 2;6(1):80. doi: 10.1038/s41541-021-00341-4. NPJ Vaccines. 2021. PMID: 34078913 Free PMC article. Review.
-
Toll-like receptor-mediated innate immune responses by recognition of the recombinant dormancy-associated Mycobacterium tuberculosis proteins Rv2659c and Rv1738.PLoS One. 2022 Sep 1;17(9):e0273517. doi: 10.1371/journal.pone.0273517. eCollection 2022. PLoS One. 2022. PMID: 36048884 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

