Comprehensive Characterization of a Next-Generation Antiviral T-Cell Product and Feasibility for Application in Immunosuppressed Transplant Patients
- PMID: 31191530
- PMCID: PMC6546853
- DOI: 10.3389/fimmu.2019.01148
Comprehensive Characterization of a Next-Generation Antiviral T-Cell Product and Feasibility for Application in Immunosuppressed Transplant Patients
Abstract
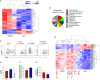
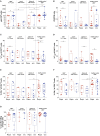
Viral infections have a major impact on morbidity and mortality of immunosuppressed solid organ transplant (SOT) patients because of missing or failure of adequate pharmacologic antiviral treatment. Adoptive antiviral T-cell therapy (AVTT), regenerating disturbed endogenous T-cell immunity, emerged as an attractive alternative approach to combat severe viral complications in immunocompromised patients. AVTT is successful in patients after hematopoietic stem cell transplantation where T-cell products (TCPs) are manufactured from healthy donors. In contrast, in the SOT setting TCPs are derived from/applied back to immunosuppressed patients. We and others demonstrated feasibility of TCP generation from SOT patients and first clinical proof-of-concept trials revealing promising data. However, the initial efficacy is frequently lost long-term, because of limited survival of transferred short-lived T-cells indicating a need for next-generation TCPs. Our recent data suggest that Rapamycin treatment during TCP manufacture, conferring partial inhibition of mTOR, might improve its composition. The aim of this study was to confirm these promising observations in a setting closer to clinical challenges and to deeply characterize the next-generation TCPs. Using cytomegalovirus (CMV) as model, our next-generation Rapamycin-treated (Rapa-)TCP showed consistently increased proportions of CD4+ T-cells as well as CD4+ and CD8+ central-memory T-cells (TCM). In addition, Rapamycin sustained T-cell function despite withdrawal of Rapamycin, showed superior T-cell viability and resistance to apoptosis, stable metabolism upon activation, preferential expansion of TCM, partial conversion of other memory T-cell subsets to TCM and increased clonal diversity. On transcriptome level, we observed a gene expression profile denoting long-lived early memory T-cells with potent effector functions. Furthermore, we successfully applied the novel protocol for the generation of Rapa-TCPs to 19/19 SOT patients in a comparative study, irrespective of their history of CMV reactivation. Moreover, comparison of paired TCPs generated before/after transplantation did not reveal inferiority of the latter despite exposition to maintenance immunosuppression post-SOT. Our data imply that the Rapa-TCPs, exhibiting longevity and sustained T-cell memory, are a reasonable treatment option for SOT patients. Based on our success to manufacture Rapa-TCPs from SOT patients under maintenance immunosuppression, now, we seek ultimate clinical proof of efficacy in a clinical study.
Keywords: Rapamycin; adoptive T-cell therapy; cytomegalovirus; immune regeneration; mTOR; solid organ transplantation.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

