Glucocorticoid Therapy of Multiple Sclerosis Patients Induces Anti-inflammatory Polarization and Increased Chemotaxis of Monocytes
- PMID: 31191554
- PMCID: PMC6549240
- DOI: 10.3389/fimmu.2019.01200
Glucocorticoid Therapy of Multiple Sclerosis Patients Induces Anti-inflammatory Polarization and Increased Chemotaxis of Monocytes
Abstract
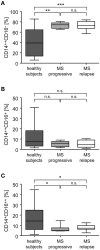
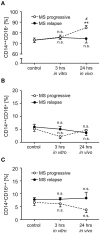
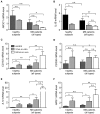
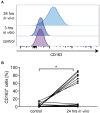
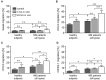
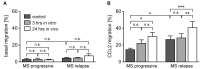
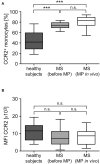
Multiple Sclerosis (MS) is an autoimmune disease of the central nervous system (CNS), characterized by the infiltration of mononuclear cells into the CNS and a subsequent inflammation of the brain. Monocytes are implicated in disease pathogenesis not only in their function as potential antigen-presenting cells involved in the local reactivation of encephalitogenic T cells but also by independent effector functions contributing to structural damage and disease progression. However, monocytes also have beneficial effects as they can exert anti-inflammatory activity and promote tissue repair. Glucocorticoids (GCs) are widely used to treat acute relapses in MS patients. They act on a variety of cell types but their exact mechanisms of action including their modulation of monocyte function are not fully understood. Here we investigated effects of the therapeutically relevant GC methylprednisolone (MP) on monocytes from healthy individuals and MS patients in vitro and in vivo. The monocyte composition in the blood was different in MS patients compared to healthy individuals, but it was only marginally affected by MP treatment. In contrast, application of MP caused a marked shift toward an anti-inflammatory monocyte phenotype in vitro and in vivo as revealed by an altered gene expression profile. Chemotaxis of monocytes toward CCL2, CCL5, and CX3CL1 was increased in MS patients compared to healthy individuals and further enhanced by MP pulse therapy. Both of these migration-promoting effects were more pronounced in MS patients with an acute relapse than in those with a progressive disease. Interestingly, the pro-migratory GC effect was independent of chemokine receptor levels as exemplified by results obtained for CCR2. Collectively, our findings suggest that GCs polarize monocytes toward an anti-inflammatory phenotype and enhance their migration into the inflamed CNS, endowing them with the capacity to suppress the pathogenic immune response.
Keywords: M2 polarization; chemokines; methylprednisolone therapy; monocytes; multiple sclerosis.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

