Serotype-Independent Protection Against Invasive Pneumococcal Infections Conferred by Live Vaccine With lgt Deletion
- PMID: 31191555
- PMCID: PMC6549034
- DOI: 10.3389/fimmu.2019.01212
Serotype-Independent Protection Against Invasive Pneumococcal Infections Conferred by Live Vaccine With lgt Deletion
Abstract
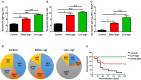
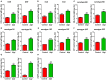
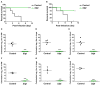
Streptococcus pneumoniae is the most common respiratory bacterial pathogen among cases of community-acquired infection in young children, older adults, and individuals with underlying medical conditions. Although capsular polysaccharide-based pneumococcal vaccines have contributed to significant decrease in invasive pneumococcal infections, these vaccines have some limitations, including limited serotype coverage, lack of effective mucosal antibody responses, and high costs. In this study, we investigated the safety and immunogenicity of a live, whole-cell pneumococcal vaccine constructed by deleting the gene for prolipoprotein diacylglyceryl transferase (lgt) from the encapsulated pneumococcal strain TIGR4 (TIGR4Δlgt) for protection against heterologous pneumococcal strains. Pneumococcal strain TIGR4 was successfully attenuated by deletion of lgt, resulting in the loss of inflammatory activity and virulence. TIGR4Δlgt colonized the nasopharynx long enough to induce strong mucosal IgA and IgG2b-dominant systemic antibody responses that were cross-reactive to heterologous pneumococcal serotypes. Finally, intranasal immunization with TIGR4Δlgt provided serotype-independent protection against pneumococcal challenge in mice. Taken together, our results suggest that TIGR4Δlgt is an avirulent and attractive broad-spectrum pneumococcal vaccine candidate. More broadly, we assert that modulation of such "master" metabolic genes represents an emerging strategy for developing more effective vaccines against numerous infectious agents.
Keywords: Lgt; Streptococcus pneumoniae; lipoprotein; live attenuated vaccine; mucosal immunity.
Figures


Similar articles
-
Intranasal Immunization with the Commensal Streptococcus mitis Confers Protective Immunity against Pneumococcal Lung Infection.Appl Environ Microbiol. 2019 Mar 6;85(6):e02235-18. doi: 10.1128/AEM.02235-18. Print 2019 Mar 15. Appl Environ Microbiol. 2019. PMID: 30683742 Free PMC article.
-
Mucosal and systemic immunization with a novel attenuated pneumococcal vaccine candidate confer serotype independent protection against Streptococcus pneumoniae in mice.Vaccine. 2014 Jul 16;32(33):4179-88. doi: 10.1016/j.vaccine.2014.05.019. Epub 2014 Jun 2. Vaccine. 2014. PMID: 24945468
-
Live attenuated Streptococcus pneumoniae strains induce serotype-independent mucosal and systemic protection in mice.Infect Immun. 2007 May;75(5):2469-75. doi: 10.1128/IAI.01972-06. Epub 2007 Mar 5. Infect Immun. 2007. PMID: 17339359 Free PMC article.
-
Understanding host immune responses to pneumococcal proteins in the upper respiratory tract to develop serotype-independent pneumococcal vaccines.Expert Rev Vaccines. 2020 Oct;19(10):959-972. doi: 10.1080/14760584.2020.1843433. Epub 2020 Nov 8. Expert Rev Vaccines. 2020. PMID: 33107359 Review.
-
Dendritic cell-targeting DNA-based nasal adjuvants for protective mucosal immunity to Streptococcus pneumoniae.Microbiol Immunol. 2017 Jun;61(6):195-205. doi: 10.1111/1348-0421.12487. Microbiol Immunol. 2017. PMID: 28463465 Free PMC article. Review.
Cited by
-
Non-capsular based immunization approaches to prevent Streptococcus pneumoniae infection.Front Cell Infect Microbiol. 2022 Sep 26;12:949469. doi: 10.3389/fcimb.2022.949469. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36225231 Free PMC article. Review.
-
A Trivalent Live Vaccine Elicits Cross-Species Protection Against Acute Otitis Media in a Murine Model.Vaccines (Basel). 2024 Dec 19;12(12):1432. doi: 10.3390/vaccines12121432. Vaccines (Basel). 2024. PMID: 39772092 Free PMC article.
-
Vaccination With the Commensal Streptococcus mitis Expressing Pneumococcal Serotype 5 Capsule Elicits IgG/IgA and Th17 Responses Against Streptococcus pneumoniae.Front Immunol. 2021 Apr 19;12:676488. doi: 10.3389/fimmu.2021.676488. eCollection 2021. Front Immunol. 2021. PMID: 33953733 Free PMC article.
-
Bacterial Vaccinations in Patients with Chronic Obstructive Pulmonary Disease.Vaccines (Basel). 2024 Feb 18;12(2):213. doi: 10.3390/vaccines12020213. Vaccines (Basel). 2024. PMID: 38400196 Free PMC article. Review.
-
Bacterial surface lipoproteins mediate epithelial microinvasion by Streptococcus pneumoniae.Infect Immun. 2024 May 7;92(5):e0044723. doi: 10.1128/iai.00447-23. Epub 2024 Apr 17. Infect Immun. 2024. PMID: 38629841 Free PMC article.
References
-
- Savulescu C, Krizova P, Lepoutre A, Mereckiene J, Vestrheim DF, Ciruela P, et al. . Effect of high-valency pneumococcal conjugate vaccines on invasive pneumococcal disease in children in SpIDnet countries: an observational multicentre study. Lancet Respir Med. (2017) 5:648–56. 10.1016/S2213-2600(17)30110-8 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

