Butterfly Pea (Clitoria ternatea), a Cyclotide-Bearing Plant With Applications in Agriculture and Medicine
- PMID: 31191573
- PMCID: PMC6546959
- DOI: 10.3389/fpls.2019.00645
Butterfly Pea (Clitoria ternatea), a Cyclotide-Bearing Plant With Applications in Agriculture and Medicine
Abstract
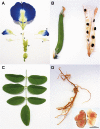
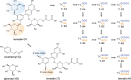
The perennial leguminous herb Clitoria ternatea (butterfly pea) has attracted significant interest based on its agricultural and medical applications, which range from use as a fodder and nitrogen fixing crop, to applications in food coloring and cosmetics, traditional medicine and as a source of an eco-friendly insecticide. In this article we provide a broad multidisciplinary review that includes descriptions of the physical appearance, distribution, taxonomy, habitat, growth and propagation, phytochemical composition and applications of this plant. Notable amongst its repertoire of chemical components are anthocyanins which give C. ternatea flowers their characteristic blue color, and cyclotides, ultra-stable macrocyclic peptides that are present in all tissues of this plant. The latter are potent insecticidal molecules and are implicated as the bioactive agents in a plant extract used commercially as an insecticide. We include a description of the genetic origin of these peptides, which interestingly involve the co-option of an ancestral albumin gene to produce the cyclotide precursor protein. The biosynthesis step in which the cyclic peptide backbone is formed involves an asparaginyl endopeptidase, of which in C. ternatea is known as butelase-1. This enzyme is highly efficient in peptide ligation and has been the focus of many recent studies on peptide ligation and cyclization for biotechnological applications. The article concludes with some suggestions for future studies on this plant, including the need to explore possible synergies between the various peptidic and non-peptidic phytochemicals.
Keywords: anthocyanins; butelase; forage crop; medicinal plant; organic pesticide; peptides.
Figures

References
-
- Abdelhamid A. M., Gabr A. A. (1993). The evaluation of new sources of fodder (Clitoria and Phillipesara) under Egyptian conditions. Arch. Anim. Nutr. 44 85–93. 10.1080/17450399309386060 - DOI
-
- Alderete-Chavez A., Guerra-Santos J. J., Cruz-Landero De la N., Brito R., Gelabert R., Endanu E., et al. (2011). Evaluation of Clitoria ternatea L. in relation with fertility in tropical soils. J. Appl. Sci. 11 1044–1048. 10.3923/jas.2011.1044.1048 - DOI
Publication types
LinkOut - more resources
Full Text Sources

