1-Aminocyclopropane-1-Carboxylic Acid Oxidase (ACO): The Enzyme That Makes the Plant Hormone Ethylene
- PMID: 31191592
- PMCID: PMC6549523
- DOI: 10.3389/fpls.2019.00695
1-Aminocyclopropane-1-Carboxylic Acid Oxidase (ACO): The Enzyme That Makes the Plant Hormone Ethylene
Abstract
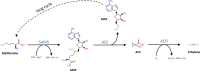
The volatile plant hormone ethylene regulates many plant developmental processes and stress responses. It is therefore crucial that plants can precisely control their ethylene production levels in space and time. The ethylene biosynthesis pathway consists of two dedicated steps. In a first reaction, S-adenosyl-L-methionine (SAM) is converted into 1-aminocyclopropane-1-carboxylic acid (ACC) by ACC-synthase (ACS). In a second reaction, ACC is converted into ethylene by ACC-oxidase (ACO). Initially, it was postulated that ACS is the rate-limiting enzyme of this pathway, directing many studies to unravel the regulation of ACS protein activity, and stability. However, an increasing amount of evidence has been gathered over the years, which shows that ACO is the rate-limiting step in ethylene production during certain dedicated processes. This implies that also the ACO protein family is subjected to a stringent regulation. In this review, we give an overview about the state-of-the-art regarding ACO evolution, functionality and regulation, with an emphasis on the transcriptional, post-transcriptional, and post-translational control. We also highlight the importance of ACO being a prime target for genetic engineering and precision breeding, in order to control plant ethylene production levels.
Keywords: 1-aminocyclopropane-1-carboxylate oxidase; ethylene biosynthesis; phylogeny; physiology; transcriptional and post-translation regulation.
Figures


Similar articles
-
The Ethylene Biosynthetic Enzymes, 1-Aminocyclopropane-1-Carboxylate (ACC) Synthase (ACS) and ACC Oxidase (ACO): The Less Explored Players in Abiotic Stress Tolerance.Biomolecules. 2024 Jan 11;14(1):90. doi: 10.3390/biom14010090. Biomolecules. 2024. PMID: 38254690 Free PMC article. Review.
-
Identification and active site analysis of the 1-aminocyclopropane-1-carboxylic acid oxidase catalysing the synthesis of ethylene in Agaricus bisporus.Biochim Biophys Acta. 2014 Jan;1840(1):120-8. doi: 10.1016/j.bbagen.2013.08.030. Epub 2013 Sep 6. Biochim Biophys Acta. 2014. PMID: 24016603
-
The regulation of ethylene biosynthesis: a complex multilevel control circuitry.New Phytol. 2021 Jan;229(2):770-782. doi: 10.1111/nph.16873. Epub 2020 Sep 12. New Phytol. 2021. PMID: 32790878 Free PMC article. Review.
-
A 1-aminocyclopropane-1-carboxylic-acid (ACC) dipeptide elicits ethylene responses through ACC-oxidase mediated substrate promiscuity.Front Plant Sci. 2022 Sep 12;13:995073. doi: 10.3389/fpls.2022.995073. eCollection 2022. Front Plant Sci. 2022. PMID: 36172554 Free PMC article.
-
Assay Methods for ACS Activity and ACS Phosphorylation by MAP Kinases In Vitro and In Vivo.Methods Mol Biol. 2017;1573:59-71. doi: 10.1007/978-1-4939-6854-1_6. Methods Mol Biol. 2017. PMID: 28293840
Cited by
-
Identification of miRNAs and Their Targets Involved in Flower and Fruit Development across Domesticated and Wild Capsicum Species.Int J Mol Sci. 2021 May 4;22(9):4866. doi: 10.3390/ijms22094866. Int J Mol Sci. 2021. PMID: 34064462 Free PMC article.
-
The nonpathogenic strain of Fusarium oxysporum FO12 induces Fe deficiency responses in cucumber (Cucumis sativus L.) plants.Planta. 2023 Feb 9;257(3):50. doi: 10.1007/s00425-023-04079-2. Planta. 2023. PMID: 36757472 Free PMC article.
-
Ethylene Acts as a Local and Systemic Signal to Mediate UV-B-Induced Nitrate Reallocation to Arabidopsis Leaves and Roots via Regulating the ERFs-NRT1.8 Signaling Module.Int J Mol Sci. 2022 Aug 13;23(16):9068. doi: 10.3390/ijms23169068. Int J Mol Sci. 2022. PMID: 36012333 Free PMC article.
-
Ethylene-Mediated Modulation of Bud Phenology, Cold Hardiness, and Hormone Biosynthesis in Peach (Prunus persica).Plants (Basel). 2021 Jun 22;10(7):1266. doi: 10.3390/plants10071266. Plants (Basel). 2021. PMID: 34206266 Free PMC article.
-
Pan-transcriptome identifying master genes and regulation network in response to drought and salt stresses in Alfalfa (Medicago sativa L.).Sci Rep. 2021 Aug 26;11(1):17203. doi: 10.1038/s41598-021-96712-x. Sci Rep. 2021. PMID: 34446782 Free PMC article.
References
-
- Adams D. O., Yang S. F. (1981). Ethylene the gaseous plant hormone: mechanism and regulation of biosynthesis. Trends Biochem. Sci. 6 161–164. 10.1016/0968-0004(81)90059-90051 - DOI
-
- Aida R., Yoshida T., Ichimura K., Goto R., Shibata M. (1998). Extension of flower longevity in transgenic torenia plants incorporating ACC oxidase transgene. Plant Sci. 138 91–101. 10.1016/S0168-9452(98)00139-133 - DOI
-
- Aik W. S., Chowdhury R., Clifton I. J., Hopkinson R. J., Leissing T., McDonough M. A., et al. (2015). Introduction to structural studies on 2-oxoglutarate-dependent oxygenases and related enzymes. RSC Metallobiol. 2015 59–94. 10.1039/9781782621959-00059 - DOI
Publication types
LinkOut - more resources
Full Text Sources

