Stroke Induces a BDNF-Dependent Improvement in Cognitive Flexibility in Aged Mice
- PMID: 31191635
- PMCID: PMC6525942
- DOI: 10.1155/2019/1460890
Stroke Induces a BDNF-Dependent Improvement in Cognitive Flexibility in Aged Mice
Abstract
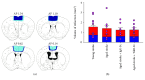
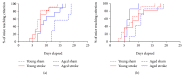
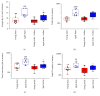
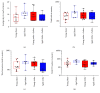
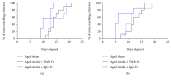
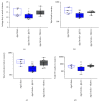
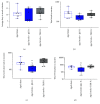
Stroke remains a leading cause of disability worldwide. Recently, we have established an animal model of stroke that results in delayed impairment in spatial memory, allowing us to better investigate cognitive deficits. Young and aged brains show different recovery profiles after stroke; therefore, we assessed aged-related differences in poststroke cognition. As neurotrophic support diminishes with age, we also investigated the involvement of brain-derived neurotrophic factor (BDNF) in these differences. Young (3-6 months old) and aged (16-21 months old) mice were trained in operant touchscreen chambers to complete a visual pairwise discrimination (VD) task. Stroke or sham surgery was induced using the photothrombotic model to induce a bilateral prefrontal cortex stroke. Five days poststroke, an additional cohort of aged stroke animals were treated with intracerebral hydrogels loaded with the BDNF decoy, TrkB-Fc. Following treatment, animals underwent the reversal and rereversal task to identify stroke-induced cognitive deficits at days 17 and 37 poststroke, respectively. Assessment of sham animals using Cox regression and log-rank analyses showed aged mice exhibit an increased impairment on VD reversal and rereversal learning compared to young controls. Stroke to young mice revealed no impairment on either task. In contrast, stroke to aged mice facilitated a significant improvement in reversal learning, which was dampened in the presence of the BDNF decoy, TrkB-Fc. In addition, aged stroke control animals required significantly less consecutive days and correction trials to master the reversal task, relative to aged shams, an effect dampened by TrkB-Fc. Our findings support age-related differences in recovery of cognitive function after stroke. Interestingly, aged stroke animals outperformed their sham counterparts, suggesting reopening of a critical window for recovery that is being mediated by BDNF.
Figures
Similar articles
-
Mice with exonic RELN deletion identified from a patient with schizophrenia have impaired visual discrimination learning and reversal learning in touchscreen operant tasks.Behav Brain Res. 2022 Jan 7;416:113569. doi: 10.1016/j.bbr.2021.113569. Epub 2021 Sep 6. Behav Brain Res. 2022. PMID: 34499931
-
Reversal Learning Deficits Associated with Increased Frontal Cortical Brain-Derived Neurotrophic Factor Tyrosine Kinase B Signaling in a Prenatal Cocaine Exposure Mouse Model.Dev Neurosci. 2016;38(5):354-364. doi: 10.1159/000452739. Epub 2016 Dec 13. Dev Neurosci. 2016. PMID: 27951531 Free PMC article.
-
Prefrontal cortex and hippocampus in behavioural flexibility and posttraumatic functional recovery: reversal learning and set-shifting in rats.Brain Res Bull. 2015 Jul;116:34-44. doi: 10.1016/j.brainresbull.2015.05.006. Epub 2015 May 29. Brain Res Bull. 2015. PMID: 26033702
-
Strain-dependent effects on acquisition and reversal of visual and spatial tasks in a rat touchscreen battery of cognition.Physiol Behav. 2015 May 15;144:26-36. doi: 10.1016/j.physbeh.2015.03.001. Epub 2015 Mar 3. Physiol Behav. 2015. PMID: 25744936
-
Post-stroke recovery: the role of activity-dependent release of brain-derived neurotrophic factor.Expert Rev Neurother. 2014 Nov;14(11):1335-44. doi: 10.1586/14737175.2014.969242. Epub 2014 Oct 16. Expert Rev Neurother. 2014. PMID: 25319267 Review.
Cited by
-
The role of the medial prefrontal cortex in cognition, ageing and dementia.Brain Commun. 2021 Jun 11;3(3):fcab125. doi: 10.1093/braincomms/fcab125. eCollection 2021 Jul. Brain Commun. 2021. PMID: 34222873 Free PMC article. Review.
-
The Gliopeptide ODN, a Ligand for the Benzodiazepine Site of GABAA Receptors, Boosts Functional Recovery after Stroke.J Neurosci. 2021 Aug 18;41(33):7148-7159. doi: 10.1523/JNEUROSCI.2255-20.2021. Epub 2021 Jul 1. J Neurosci. 2021. PMID: 34210784 Free PMC article.
-
Materials to Promote Recovery After Stroke.Curr Opin Biomed Eng. 2020 Jun;14:9-17. doi: 10.1016/j.cobme.2020.04.002. Epub 2020 Apr 13. Curr Opin Biomed Eng. 2020. PMID: 32524039 Free PMC article.
-
Rodent Models of Post-Stroke Dementia.Int J Mol Sci. 2022 Sep 15;23(18):10750. doi: 10.3390/ijms231810750. Int J Mol Sci. 2022. PMID: 36142661 Free PMC article. Review.
-
Elevated microglial oxidative phosphorylation and phagocytosis stimulate post-stroke brain remodeling and cognitive function recovery in mice.Commun Biol. 2022 Jan 11;5(1):35. doi: 10.1038/s42003-021-02984-4. Commun Biol. 2022. PMID: 35017668 Free PMC article.
References
-
- Ballard C., Rowan E., Stephens S., Kalaria R., Kenny R. A. Prospective follow-up study between 3 and 15 months after stroke: improvements and decline in cognitive function among dementia-free stroke survivors >75 years of age. Stroke. 2003;34(10):2440–2444. doi: 10.1161/01.STR.0000089923.29724.CE. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

