GSK-3 β at the Intersection of Neuronal Plasticity and Neurodegeneration
- PMID: 31191636
- PMCID: PMC6525914
- DOI: 10.1155/2019/4209475
GSK-3 β at the Intersection of Neuronal Plasticity and Neurodegeneration
Abstract
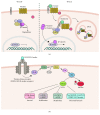
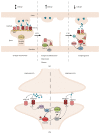
In neurons, Glycogen Synthase Kinase-3β (GSK-3β) has been shown to regulate various critical processes underlying structural and functional synaptic plasticity. Mouse models with neuron-selective expression or deletion of GSK-3β present behavioral and cognitive abnormalities, positioning this protein kinase as a key signaling molecule in normal brain functioning. Furthermore, mouse models with defective GSK-3β activity display distinct structural and behavioral abnormalities, which model some aspects of different neurological and neuropsychiatric disorders. Equalizing GSK-3β activity in these mouse models by genetic or pharmacological interventions is able to rescue some of these abnormalities. Thus, GSK-3β is a relevant therapeutic target for the treatment of many brain disorders. Here, we provide an overview of how GSK-3β is regulated in physiological synaptic plasticity and how aberrant GSK-3β activity contributes to the development of dysfunctional synaptic plasticity in neuropsychiatric and neurodegenerative disorders.
Figures
Similar articles
-
Dysregulation of miRNAs Levels in Glycogen Synthase Kinase-3β Overexpressing Mice and the Role of miR-221-5p in Synaptic Function.Neuroscience. 2022 May 10;490:287-295. doi: 10.1016/j.neuroscience.2022.03.024. Epub 2022 Mar 21. Neuroscience. 2022. PMID: 35331845
-
Control of neuronal excitability by GSK-3beta: Epilepsy and beyond.Biochim Biophys Acta Mol Cell Res. 2020 Sep;1867(9):118745. doi: 10.1016/j.bbamcr.2020.118745. Epub 2020 May 23. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 32450268 Review.
-
Chronic fluoride exposure induces neuronal apoptosis and impairs neurogenesis and synaptic plasticity: Role of GSK-3β/β-catenin pathway.Chemosphere. 2019 Jan;214:430-435. doi: 10.1016/j.chemosphere.2018.09.095. Epub 2018 Sep 17. Chemosphere. 2019. PMID: 30273876
-
GSK-3β deletion in dentate gyrus excitatory neuron impairs synaptic plasticity and memory.Sci Rep. 2017 Jul 18;7(1):5781. doi: 10.1038/s41598-017-06173-4. Sci Rep. 2017. PMID: 28720858 Free PMC article.
-
Glycogen Synthase Kinase-3β as a Putative Therapeutic Target for Bipolar Disorder.Curr Drug Metab. 2018;19(8):663-673. doi: 10.2174/1389200219666171227203737. Curr Drug Metab. 2018. PMID: 29283064 Review.
Cited by
-
A Crosstalk between the Biorhythms and Gatekeepers of Longevity: Dual Role of Glycogen Synthase Kinase-3.Biochemistry (Mosc). 2021 Apr;86(4):433-448. doi: 10.1134/S0006297921040052. Biochemistry (Mosc). 2021. PMID: 33941065 Free PMC article. Review.
-
GSK-3β inhibitor TWS119 promotes neuronal differentiation after hypoxic-ischemic brain damage in neonatal rats.Neuroreport. 2024 Feb 7;35(3):200-207. doi: 10.1097/WNR.0000000000002006. Epub 2024 Jan 31. Neuroreport. 2024. PMID: 38305107 Free PMC article.
-
Molecular Mechanisms of Memory Consolidation That Operate During Sleep.Front Mol Neurosci. 2021 Nov 18;14:767384. doi: 10.3389/fnmol.2021.767384. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34867190 Free PMC article. Review.
-
Neurotrophic effects of intermittent fasting, calorie restriction and exercise: a review and annotated bibliography.Front Aging. 2023 Jun 2;4:1161814. doi: 10.3389/fragi.2023.1161814. eCollection 2023. Front Aging. 2023. PMID: 37334045 Free PMC article. Review.
-
Olfactory three needle regulates the proliferation of olfactory bulb neural stem cells and ameliorates brain injury after subarachnoid hemorrhage by regulating Wnt/β-catenin signaling.Heliyon. 2024 Mar 25;10(7):e28551. doi: 10.1016/j.heliyon.2024.e28551. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38596082 Free PMC article.
References
-
- Lisman J. Glutamatergic synapses are structurally and biochemically complex because of multiple plasticity processes: long-term potentiation, long-term depression, short-term potentiation and scaling. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1715, article 20160260) doi: 10.1098/rstb.2016.0260. - DOI - PMC - PubMed
-
- Embi N., Rylatt D. B., Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. European Journal of Biochemistry. 1980;107(2):519–527. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

