Generation and miRNA Characterization of Equine Induced Pluripotent Stem Cells Derived from Fetal and Adult Multipotent Tissues
- PMID: 31191664
- PMCID: PMC6525926
- DOI: 10.1155/2019/1393791
Generation and miRNA Characterization of Equine Induced Pluripotent Stem Cells Derived from Fetal and Adult Multipotent Tissues
Abstract
Introduction: Pluripotent stem cells are believed to have greater clinical potential than mesenchymal stem cells due to their ability to differentiate into almost any cell type of an organism, and since 2006, the generation of patient-specific induced pluripotent stem cells (iPSCs) has become possible in multiple species.
Objectives: We hypothesize that different cell types respond differently to the reprogramming process; thus, the goals of this study were to isolate and characterize equine adult and fetal cells and induce these cells to pluripotency for future regenerative and translational purposes.
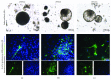
Methods: Adult equine fibroblasts (eFibros) and mesenchymal cells derived from the bone marrow (eBMmsc), adipose tissue (eADmsc), and umbilical cord tissue (eUCmsc) were isolated, their multipotency was characterized, and the cells were induced in vitro into pluripotency (eiPSCs). eiPSCs were generated through a lentiviral system using the factors OCT4, SOX2, c-MYC, and KLF4. The morphology and in vitro pluripotency maintenance potential (alkaline phosphatase detection, embryoid body formation, in vitro spontaneous differentiation, and expression of pluripotency markers) of the eiPSCs were characterized. Additionally, a miRNA profile analysis of the mesenchymal and eiPSCs was performed.
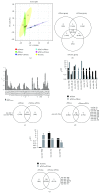
Results: Multipotent cells were successfully isolated, but the eBMmsc failed to generate eiPSCs. The eADmsc-, eUCmsc-, and eFibros-derived iPSCs were positive for alkaline phosphatase, OCT4 and NANOG, were exclusively dependent on bFGF, and formed embryoid bodies. The miRNA profile revealed a segregated pattern between the eiPSCs and multipotent controls: the levels of miR-302/367 and the miR-92 family were increased in the eiPSCs, while the levels of miR-23, miR-27, and miR-30, as well as the let-7 family were increased in the nonpluripotent cells.
Conclusions: We were able to generate bFGF-dependent iPSCs from eADmsc, eUCmsc, and eFibros with human OSKM, and the miRNA profile revealed that clonal lines may respond differently to the reprogramming process.
Figures


Similar articles
-
In vitro induced pluripotency from urine-derived cells in porcine.World J Stem Cells. 2022 Mar 26;14(3):231-244. doi: 10.4252/wjsc.v14.i3.231. World J Stem Cells. 2022. PMID: 35432738 Free PMC article.
-
In Vitro Induction of Pluripotency from Equine Fibroblasts in 20% or 5% Oxygen.Stem Cells Int. 2020 Nov 26;2020:8814989. doi: 10.1155/2020/8814989. eCollection 2020. Stem Cells Int. 2020. PMID: 33456472 Free PMC article.
-
MicroRNA characterization in equine induced pluripotent stem cells.PLoS One. 2018 Dec 3;13(12):e0207074. doi: 10.1371/journal.pone.0207074. eCollection 2018. PLoS One. 2018. PMID: 30507934 Free PMC article.
-
Embryonic stem cell microRNAs: defining factors in induced pluripotent (iPS) and cancer (CSC) stem cells?Curr Stem Cell Res Ther. 2009 Sep;4(3):168-77. doi: 10.2174/157488809789057400. Curr Stem Cell Res Ther. 2009. PMID: 19492978 Review.
-
Current understanding and future perspectives of the roles of sirtuins in the reprogramming and differentiation of pluripotent stem cells.Exp Biol Med (Maywood). 2018 Mar;243(6):563-575. doi: 10.1177/1535370218759636. Exp Biol Med (Maywood). 2018. PMID: 29557214 Free PMC article. Review.
Cited by
-
Generation of induced pluripotent stem cells from large domestic animals.Stem Cell Res Ther. 2020 Jun 25;11(1):247. doi: 10.1186/s13287-020-01716-5. Stem Cell Res Ther. 2020. PMID: 32586372 Free PMC article.
-
In vitro induced pluripotency from urine-derived cells in porcine.World J Stem Cells. 2022 Mar 26;14(3):231-244. doi: 10.4252/wjsc.v14.i3.231. World J Stem Cells. 2022. PMID: 35432738 Free PMC article.
-
Induced pluripotent stem cells throughout the animal kingdom: Availability and applications.World J Stem Cells. 2019 Aug 26;11(8):491-505. doi: 10.4252/wjsc.v11.i8.491. World J Stem Cells. 2019. PMID: 31523369 Free PMC article. Review.
-
Induced pluripotent stem cells from farm animals.J Anim Sci. 2020 Nov 1;98(11):skaa343. doi: 10.1093/jas/skaa343. J Anim Sci. 2020. PMID: 33098420 Free PMC article. Review.
-
Emerging Contributions of Pluripotent Stem Cells to Reproductive Technologies in Veterinary Medicine.J Dev Biol. 2024 May 7;12(2):14. doi: 10.3390/jdb12020014. J Dev Biol. 2024. PMID: 38804434 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

