Recent Updates on Induced Pluripotent Stem Cells in Hematological Disorders
- PMID: 31191673
- PMCID: PMC6525795
- DOI: 10.1155/2019/5171032
Recent Updates on Induced Pluripotent Stem Cells in Hematological Disorders
Abstract
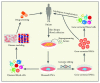
Over the past decade, enormous progress has been made in the field of induced pluripotent stem cells (iPSCs). Patients' somatic cells such as skin fibroblasts or blood cells can be used to generate disease-specific pluripotent stem cells, which have unlimited proliferation and can differentiate into all cell types of the body. Human iPSCs offer great promises and opportunities for treatments of degenerative diseases and studying disease pathology and drug screening. So far, many iPSC-derived disease models have led to the discovery of novel pathological mechanisms as well as new drugs in the pipeline that have been tested in the iPSC-derived cells for efficacy and potential toxicities. Furthermore, recent advances in genome editing technology in combination with the iPSC technology have provided a versatile platform for studying stem cell biology and regenerative medicine. In this review, an overview of iPSCs, patient-specific iPSCs for disease modeling and drug screening, applications of iPSCs and genome editing technology in hematological disorders, remaining challenges, and future perspectives of iPSCs in hematological diseases will be discussed.
Figures
Similar articles
-
Application of induced pluripotent stem cell technology for the investigation of hematological disorders.Adv Biol Regul. 2019 Jan;71:19-33. doi: 10.1016/j.jbior.2018.10.001. Epub 2018 Oct 10. Adv Biol Regul. 2019. PMID: 30341008 Review.
-
Induced pluripotent stem cell technology: a decade of progress.Nat Rev Drug Discov. 2017 Feb;16(2):115-130. doi: 10.1038/nrd.2016.245. Epub 2016 Dec 16. Nat Rev Drug Discov. 2017. PMID: 27980341 Free PMC article. Review.
-
Exploiting urine-derived induced pluripotent stem cells for advancing precision medicine in cell therapy, disease modeling, and drug testing.J Biomed Sci. 2024 May 9;31(1):47. doi: 10.1186/s12929-024-01035-4. J Biomed Sci. 2024. PMID: 38724973 Free PMC article. Review.
-
Ten years of progress and promise of induced pluripotent stem cells: historical origins, characteristics, mechanisms, limitations, and potential applications.PeerJ. 2018 May 11;6:e4370. doi: 10.7717/peerj.4370. eCollection 2018. PeerJ. 2018. PMID: 29770269 Free PMC article.
-
Is Human-induced Pluripotent Stem Cell the Best Optimal?Chin Med J (Engl). 2018 Apr 5;131(7):852-856. doi: 10.4103/0366-6999.228231. Chin Med J (Engl). 2018. PMID: 29578130 Free PMC article. Review.
Cited by
-
Therapeutic effects of stem cells in different body systems, a novel method that is yet to gain trust: A comprehensive review.Bosn J Basic Med Sci. 2021 Dec 1;21(6):672-701. doi: 10.17305/bjbms.2021.5508. Bosn J Basic Med Sci. 2021. PMID: 34255619 Free PMC article. Review.
-
In vitro erythropoiesis: the emerging potential of induced pluripotent stem cells (iPSCs).Blood Sci. 2024 Dec 26;7(1):e00215. doi: 10.1097/BS9.0000000000000215. eCollection 2025 Jan. Blood Sci. 2024. PMID: 39726795 Free PMC article. Review.
-
Utilization of Modified Induced Pluripotent Stem Cells as the Advance Therapy of Glaucoma: A Systematic Review.Clin Ophthalmol. 2022 Aug 29;16:2851-2859. doi: 10.2147/OPTH.S372114. eCollection 2022. Clin Ophthalmol. 2022. PMID: 36061629 Free PMC article. Review.
-
Advances in Adoptive Cell Therapy Using Induced Pluripotent Stem Cell-Derived T Cells.Front Immunol. 2021 Sep 28;12:759558. doi: 10.3389/fimmu.2021.759558. eCollection 2021. Front Immunol. 2021. PMID: 34650571 Free PMC article. Review.
-
Differentiation of human induced pluripotent stem cells into erythroid cells.Stem Cell Res Ther. 2020 Nov 16;11(1):483. doi: 10.1186/s13287-020-01998-9. Stem Cell Res Ther. 2020. PMID: 33198819 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

