Radionuclide Imaging of Atherothrombotic Diseases
- PMID: 31191793
- PMCID: PMC6561494
- DOI: 10.1007/s12410-019-9491-7
Radionuclide Imaging of Atherothrombotic Diseases
Abstract
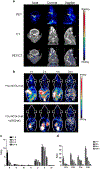
Purpose of review: A variety of approaches and molecular targets have emerged in recent years for radionuclide-based imaging of atherosclerosis and vulnerable plaque using single photon emission computed tomography (SPECT) and positron emission tomography (PET), with numerous methods focused on characterizing the mechanisms underlying plaque progression and rupture. This review highlights the ongoing developments in both the preclinical and clinical environment for radionuclide imaging of atherosclerosis and atherothrombosis.
Recent findings: Numerous physiological processes responsible for the evolution of high-risk atherosclerotic plaque, such as inflammation, thrombosis, angiogenesis, and microcalcification, have been shown to be feasible targets for SPECT and PET imaging. For each physiological process, specific molecular markers have been identified that allow for sensitive non-invasive detection and characterization of atherosclerotic plaque.
Summary: The capabilities of SPECT and PET imaging continue to evolve for physiological evaluation of atherosclerosis. This review summarizes the latest developments related to radionuclide imaging of atherothrombotic diseases.
Keywords: PET; SPECT; atherosclerosis; atherothrombosis; molecular imaging; radionuclide imaging.
Conflict of interest statement
Conflict of Interest Dr. Stacy has no conflicts of interest to declare for this work.
Figures

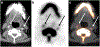
Similar articles
-
A Clinical Role of PET in Atherosclerosis and Vulnerable Plaques?Semin Nucl Med. 2020 Jul;50(4):311-318. doi: 10.1053/j.semnuclmed.2020.02.017. Epub 2020 Mar 20. Semin Nucl Med. 2020. PMID: 32540028 Review.
-
Nuclear Molecular Imaging for Vulnerable Atherosclerotic Plaques.Korean J Radiol. 2015 Sep-Oct;16(5):955-66. doi: 10.3348/kjr.2015.16.5.955. Epub 2015 Aug 21. Korean J Radiol. 2015. PMID: 26357491 Free PMC article. Review.
-
Molecular Imaging and Non-molecular Imaging of Atherosclerotic Plaque Thrombosis.Front Cardiovasc Med. 2021 Jul 5;8:692915. doi: 10.3389/fcvm.2021.692915. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34291095 Free PMC article. Review.
-
Emerging Molecular Targets for Imaging of Atherosclerotic Plaque using Positron Emission Tomography.Curr Radiopharm. 2021;14(3):173-183. doi: 10.2174/1874471013666200505102353. Curr Radiopharm. 2021. PMID: 32368992 Review.
-
Targeted PET/CT imaging of vulnerable atherosclerotic plaques: microcalcification with sodium fluoride and inflammation with fluorodeoxyglucose.Curr Cardiol Rep. 2013 Jun;15(6):364. doi: 10.1007/s11886-013-0364-4. Curr Cardiol Rep. 2013. PMID: 23605466 Review.
Cited by
-
Molecular Imaging of Lower Extremity Peripheral Arterial Disease: An Emerging Field in Nuclear Medicine.Front Med (Lausanne). 2022 Jan 12;8:793975. doi: 10.3389/fmed.2021.793975. eCollection 2021. Front Med (Lausanne). 2022. PMID: 35096884 Free PMC article.
-
Imaging of Thromboinflammation by Multispectral 19F MRI.Int J Mol Sci. 2025 Mar 10;26(6):2462. doi: 10.3390/ijms26062462. Int J Mol Sci. 2025. PMID: 40141106 Free PMC article. Review.
-
Clinical Applications for Radiotracer Imaging of Lower Extremity Peripheral Arterial Disease and Critical Limb Ischemia.Mol Imaging Biol. 2020 Apr;22(2):245-255. doi: 10.1007/s11307-019-01425-3. Mol Imaging Biol. 2020. PMID: 31482412 Free PMC article. Review.
-
Multi-Modality Imaging of Atheromatous Plaques in Peripheral Arterial Disease: Integrating Molecular and Imaging Markers.Int J Mol Sci. 2023 Jul 5;24(13):11123. doi: 10.3390/ijms241311123. Int J Mol Sci. 2023. PMID: 37446302 Free PMC article. Review.
-
Vessel-by-vessel analysis of lower extremity 18F-NaF PET/CT imaging quantifies diabetes- and chronic kidney disease-induced active microcalcification in patients with peripheral arterial disease.EJNMMI Res. 2023 Jan 17;13(1):3. doi: 10.1186/s13550-023-00951-0. EJNMMI Res. 2023. PMID: 36648583 Free PMC article.
References
-
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. - PubMed
-
- Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arter Thromb Vasc Biol. 2005;25:2054–61. - PubMed
-
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
