Frankincense essential oil suppresses melanoma cancer through down regulation of Bcl-2/Bax cascade signaling and ameliorates heptotoxicity via phase I and II drug metabolizing enzymes
- PMID: 31191820
- PMCID: PMC6544398
- DOI: 10.18632/oncotarget.26930
Frankincense essential oil suppresses melanoma cancer through down regulation of Bcl-2/Bax cascade signaling and ameliorates heptotoxicity via phase I and II drug metabolizing enzymes
Erratum in
-
Correction: Frankincense essential oil suppresses melanoma cancer through down regulation of Bcl-2/Bax cascade signaling and ameliorates heptotoxicity via phase I and II drug metabolizing enzymes.Oncotarget. 2020 Jun 9;11(23):2259-2261. doi: 10.18632/oncotarget.27540. eCollection 2020 Jun 9. Oncotarget. 2020. PMID: 32577169 Free PMC article.
Abstract
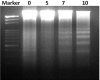
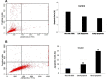
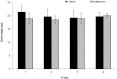
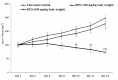
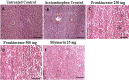
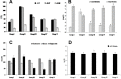
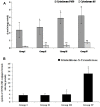
Melanoma is a deadly form of malignancy and according to the World Health Organization 132,000 new cases of melanoma are diagnosed worldwide each year. Surgical resection and chemo/drug treatments opted for early and late stage of melanoma respectively, however detrimental post surgical and chemotherapy consequences are inevitable. Noticeably melanoma drug treatments are associated with liver injuries such as hepatitis and cholestasis which are very common. Alleviation of these clinical manifestations with better treatment options would enhance prognosis status and patients survival. Natural products which induce cytotoxicity with minimum side effects are of interest to achieve high therapeutic efficiency. In this study we investigated anti-melanoma and hepatoprotective activities of frankincense essential oil (FEO) in both in vitro and in vivo models. Pretreatment with FEO induce a significant (p < 0.05) dose-dependent reduction in the cell viability of mouse (B16-F10) and human melanoma (FM94) but not in the normal human epithelial melanocytes (HNEM). Immunoblot analysis showed that FEO induces down regulation of Bcl-2 and up regulation of BAX in B16-F10 cells whereas in FM94 cells FEO induced dose-dependent cleavage of caspase 3, caspase 9 and PARP. Furthermore, FEO (10 μg/ml) treatment down regulated MCL1 in a time-dependent manner in FM94 cells. In vivo toxicity analysis reveals that weekly single dose of FEO (1200 mg/kg body weight) did not elicit detrimental effect on body weight during four weeks of experimental period. Histology of tissue sections also indicated that there were no observable histopathologic differences in the brain, heart, liver, and kidney compare to control groups. FEO (300 and 600 mg/kg body weight) treatments significantly reduced the tumor burden in C57BL/6 mice melanoma model. Acetaminophen (750 mg/kg body weight) was used to induce hepatic injury in Swiss albino mice. Pre treatment with FEO (250 and 500 mg/kg body weight) for seven days retained hematology (complete blood count), biochemical parameters (AST, ALT, ALK, total bilirubin, total protein, glucose, albumin/globulin ratio, cholesterol and triglyceride), and the level of phase I and II drug metabolizing enzymes (cytochrome P450, cytochromeb5, glutathione-S-transferase) which were obstructed by the administration of acetaminophen. Further liver histology showed that FEO treatments reversed the damages (central vein dilation, hemorrhage, and nuclei condensation) caused by acetaminophen. In conclusion, FEO elicited marked anti-melanoma in both in vitro and in vivo with a significant heptoprotection.
Keywords: apoptosis; essential oil; frankincense; melanoma; tumor remission.
Conflict of interest statement
CONFLICTS OF INTEREST Authors declare that there is no conflicts of interest.
Figures




Similar articles
-
Anti-Tumor Potential of Frankincense Essential Oil and Its Nano-Formulation in Breast Cancer: An In Vivo and In Vitro Study.Pharmaceutics. 2025 Mar 27;17(4):426. doi: 10.3390/pharmaceutics17040426. Pharmaceutics. 2025. PMID: 40284420 Free PMC article.
-
Correction: Frankincense essential oil suppresses melanoma cancer through down regulation of Bcl-2/Bax cascade signaling and ameliorates heptotoxicity via phase I and II drug metabolizing enzymes.Oncotarget. 2020 Jun 9;11(23):2259-2261. doi: 10.18632/oncotarget.27540. eCollection 2020 Jun 9. Oncotarget. 2020. PMID: 32577169 Free PMC article.
-
Capsaicin induced apoptosis of B16-F10 melanoma cells through down-regulation of Bcl-2.Food Chem Toxicol. 2007 May;45(5):708-15. doi: 10.1016/j.fct.2006.10.011. Epub 2006 Oct 27. Food Chem Toxicol. 2007. PMID: 17306913
-
Ethanol extract of Ilex hainanensis Merr. exhibits anti-melanoma activity by induction of G1/S cell-cycle arrest and apoptosis.Chin J Integr Med. 2018 Jan;24(1):47-55. doi: 10.1007/s11655-017-2544-8. Epub 2017 Jul 25. Chin J Integr Med. 2018. PMID: 28741062
-
Natural Products for Melanoma Therapy: From Traditional Medicine to Modern Drug Discovery.Plants (Basel). 2025 Mar 18;14(6):951. doi: 10.3390/plants14060951. Plants (Basel). 2025. PMID: 40265853 Free PMC article. Review.
Cited by
-
Anti-Tumor Potential of Frankincense Essential Oil and Its Nano-Formulation in Breast Cancer: An In Vivo and In Vitro Study.Pharmaceutics. 2025 Mar 27;17(4):426. doi: 10.3390/pharmaceutics17040426. Pharmaceutics. 2025. PMID: 40284420 Free PMC article.
-
Biological Activity of Some Aromatic Plants and Their Metabolites, with an Emphasis on Health-Promoting Properties.Molecules. 2020 May 27;25(11):2478. doi: 10.3390/molecules25112478. Molecules. 2020. PMID: 32471063 Free PMC article. Review.
-
Unlocking the Anticancer Potential of Frankincense Essential Oils (FEOs) Through Nanotechnology: A Review.Mol Biotechnol. 2024 Nov;66(11):3013-3024. doi: 10.1007/s12033-023-00918-5. Epub 2023 Nov 1. Mol Biotechnol. 2024. PMID: 37914864 Review.
-
Hydroxypropyl Cellulose Hydrogel Containing Origanum vulgare ssp. hirtum Essential-Oil-Loaded Polymeric Micelles for Enhanced Treatment of Melanoma.Gels. 2024 Sep 29;10(10):627. doi: 10.3390/gels10100627. Gels. 2024. PMID: 39451280 Free PMC article.
-
Essential Oils and Their Main Chemical Components: The Past 20 Years of Preclinical Studies in Melanoma.Cancers (Basel). 2020 Sep 16;12(9):2650. doi: 10.3390/cancers12092650. Cancers (Basel). 2020. PMID: 32948083 Free PMC article. Review.
References
-
- American Cancer Society Cancer Facts and Figures 2018 Atlanta: American Cancer Society; 2019.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

