How mithramycin stereochemistry dictates its structure and DNA binding function
- PMID: 31191864
- PMCID: PMC6533888
- DOI: 10.1039/c9md00100j
How mithramycin stereochemistry dictates its structure and DNA binding function
Abstract
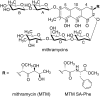
An aureolic acid natural product mithramycin (MTM) has been known for its potent antineoplastic properties. MTM inhibits cell growth by binding in the minor groove of double-stranded DNA as a dimer, in which the two molecules of MTM are coordinated to each other through a divalent metal ion. A crystal structure of an MTM analogue, MTM SA-Phe, in the active metal ion-coordinated dimeric form demonstrates how the stereochemical features of MTM define the helicity of the dimeric scaffold for its binding to a right-handed DNA double helix. We also show crystallographically and biochemically that MTM, but not MTM SA-Phe, can be inactivated by boric acid through formation of a large macrocyclic species, in which two molecules of MTM are crosslinked to each other through 3-side chain-boron-sugar intermolecular bonds. We discuss these structural and biochemical properties in the context of MTM biosynthesis and the design of MTM analogues as anticancer therapeutics.
Figures
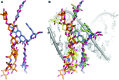
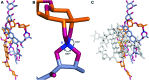
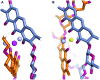
Similar articles
-
Dimerization and DNA recognition rules of mithramycin and its analogues.J Inorg Biochem. 2016 Mar;156:40-7. doi: 10.1016/j.jinorgbio.2015.12.011. Epub 2015 Dec 18. J Inorg Biochem. 2016. PMID: 26760230 Free PMC article.
-
Structures of mithramycin analogues bound to DNA and implications for targeting transcription factor FLI1.Nucleic Acids Res. 2016 Oct 14;44(18):8990-9004. doi: 10.1093/nar/gkw761. Epub 2016 Sep 1. Nucleic Acids Res. 2016. PMID: 27587584 Free PMC article.
-
Inhibition of c-src transcription by mithramycin: structure-activity relationships of biosynthetically produced mithramycin analogues using the c-src promoter as target.Biochemistry. 2003 Jul 15;42(27):8313-24. doi: 10.1021/bi034091z. Biochemistry. 2003. PMID: 12846580
-
The antitumor antibiotic mithramycin: new advanced approaches in modification and production.Appl Microbiol Biotechnol. 2020 Sep;104(18):7701-7721. doi: 10.1007/s00253-020-10782-x. Epub 2020 Jul 20. Appl Microbiol Biotechnol. 2020. PMID: 32686008 Review.
-
Mithramycin, an agent for developing new therapeutic drugs for neurodegenerative diseases.J Pharmacol Sci. 2013;122(4):251-6. doi: 10.1254/jphs.13r02cp. Epub 2013 Jul 30. J Pharmacol Sci. 2013. PMID: 23902990 Review.
Cited by
-
Discovery of a Cryptic Intermediate in Late Steps of Mithramycin Biosynthesis.Angew Chem Int Ed Engl. 2020 Jan 7;59(2):826-832. doi: 10.1002/anie.201910241. Epub 2019 Nov 27. Angew Chem Int Ed Engl. 2020. PMID: 31702856 Free PMC article.
-
The Position of Indole Methylation Controls the Structure, DNA Binding, and Cellular Functions of Mithramycin SA-Trp Analogues.Chembiochem. 2025 May 27;26(10):e202401084. doi: 10.1002/cbic.202401084. Epub 2025 May 6. Chembiochem. 2025. PMID: 40246689
-
Practical hints and tips for solution of pseudo-merohedric twins: three case studies.Acta Crystallogr E Crystallogr Commun. 2021 Apr 9;77(Pt 5):452-465. doi: 10.1107/S205698902100342X. eCollection 2021 May 1. Acta Crystallogr E Crystallogr Commun. 2021. PMID: 34026247 Free PMC article.
-
Mithramycin delivery systems to develop effective therapies in sarcomas.J Nanobiotechnology. 2021 Sep 6;19(1):267. doi: 10.1186/s12951-021-01008-x. J Nanobiotechnology. 2021. PMID: 34488783 Free PMC article.
-
Mithplatins: Mithramycin SA-Pt(II) Complex Conjugates for the Treatment of Platinum-Resistant Ovarian Cancers.ChemMedChem. 2023 Feb 1;18(3):e202200368. doi: 10.1002/cmdc.202200368. Epub 2022 Nov 22. ChemMedChem. 2023. PMID: 36342449 Free PMC article.
References
-
- Ryan W. G., Schwartz T. B., Northrop G. JAMA, J. Am. Med. Assoc. 1970;213:1153–1157. - PubMed
-
- Kennedy B. J. J. Urol. 1972;107:429–432. - PubMed
-
- Ream N. W., Perlia C. P., Wolter J., Taylor, 3rd S. G. JAMA, J. Am. Med. Assoc. 1968;204:1030–1036. - PubMed
-
- Elias E. G., Evans J. T. J. Bone Jt. Surg. 1972;54:1730–1736. - PubMed
-
- Kennedy B. J., Torkelson J. L. Med. Pediatr. Oncol. 1995;24:327–328. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

