Brønsted acid catalysis - the effect of 3,3'-substituents on the structural space and the stabilization of imine/phosphoric acid complexes
- PMID: 31191877
- PMCID: PMC6540909
- DOI: 10.1039/c9sc01044k
Brønsted acid catalysis - the effect of 3,3'-substituents on the structural space and the stabilization of imine/phosphoric acid complexes
Abstract
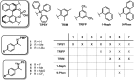
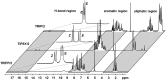
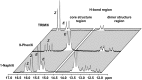
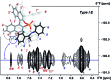
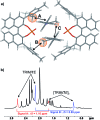
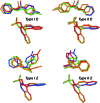
BINOL derived chiral phosphoric acids (CPAs) are widely known for their high selectivity. Numerous 3,3'-substituents are used for a variety of stereoselective reactions and theoretical models of their effects are provided. However, experimental data about the structural space of CPA complexes in solution is extremely rare and so far restricted to NMR investigations of binary TRIP/imine complexes featuring two E- and two Z-imine conformations. Therefore, in this paper the structural space of 16 CPA/imine binary complexes is screened and 8 of them are investigated in detail by NMR. For the first time dimers of CPA/imine complexes in solution were experimentally identified, which show an imine position similar to the transition state in transfer hydrogenations. Furthermore, our experimental and computational data revealed an astonishing invariance of the four core structures regardless of the different steric and electronic properties of the 3,3'-substituent. However, a significant variation of E/Z-ratios is observed, demonstrating a strong influence of the 3,3'-substituents on the stabilization of the imine in the complexes. These experimental E/Z-ratios cannot be reproduced by calculations commonly applied for mechanistic studies, despite extensive conformational scans and treatment of the electronic structure at a high level of theory with various implicit solvent corrections. Thus, these first detailed experimental data about the structural space and influence of the 3,3'-substituent on the energetics of CPA/imine complexes can serve as basis to validate and improve theoretical predictive models.
Figures





References
-
- Parmar D., Sugiono E., Raja S., Rueping M. Chem. Rev. 2014;114:9047–9153. - PubMed
-
- Parmar D., Sugiono E., Raja S., Rueping M. Chem. Rev. 2017;117:10608–10620. - PubMed
-
- Akiyama T., Itoh J., Yokota K., Fuchibe K. Angew. Chem., Int. Ed. 2004;43:1566–1568. - PubMed
-
- Uraguchi D., Terada M. J. Am. Chem. Soc. 2004;126:5356–5357. - PubMed
-
- Rueping M., Sugiono E., Azap C., Theissmann T., Bolte M. Org. Lett. 2005;7:3781–3783. - PubMed
LinkOut - more resources
Full Text Sources

