Self-promoted and stereospecific formation of N-glycosides
- PMID: 31191886
- PMCID: PMC6540880
- DOI: 10.1039/c9sc00857h
Self-promoted and stereospecific formation of N-glycosides
Abstract
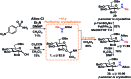
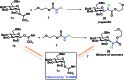
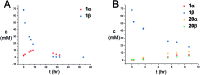
A stereoselective and self-promoted glycosylation for the synthesis of various N-glycosides and glycosyl sulfonamides from trichloroacetimidates is presented. No additional catalysts or promoters are needed in what is essentially a two-component reaction. When α-glucosyl trichloroacetimidates are employed, the reaction resulted in the stereospecific formation of the corresponding β-N-glucosides in high yields at ambient conditions. On the other hand, when equatorial glucosyl donors were used, the stereospecificity decreased and resulted in a mixture of anomers. By NMR-studies, it was concluded that this decrease in stereospecificity was due to an, until now, unpresented anomerization of the trichloroacetimidate under the very mildly acidic conditions. The mechanism and kinetics of the glycosylations have been studied by NMR-experiments, which gave an insight into the activation of trichloroacetimidates, suggesting an SNi-like mechanism involving ion pairs. The scope of glycosyl donors and sulfonamides was found to be very broad including popular N-protective groups and common glycosyl donors of various reactivity. Peracetylated GlcNAc trichloroacetimidate could be used without the need for any promotors or additives and a tyrosine side chain was glycosylated as an N-glycosyl carbamate. The N-carbamates and the N-sulfonyl groups functioned as orthogonal protective groups of the N-glycoside and hence allowed further N-functionalization without risking mutarotation of the N-glycoside. The N-glycosylation was also performed on a gram scale, without a drop in stereoselectivity nor yield.
Figures



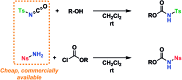




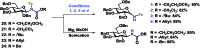
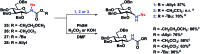
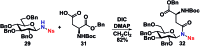



References
-
- Nielsen M. M., Pedersen C. M. Chem. Rev. 2018;118:8285–8358. - PubMed
-
- Schmidt R. R., Michel J. Angew. Chem., Int. Ed. Engl. 1980;19:731–732.
-
- Schmidt R. R., Gaden H., Jatzke H. Tetrahedron Lett. 1990;31:327–329.
-
- Urban F. J., Moore B. S., Breitenbach R. Tetrahedron Lett. 1990;31:4421–4424.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

