Identification of isoform-selective hydroxamic acid derivatives that potently reactivate HIV from latency
- PMID: 31191911
- PMCID: PMC6543484
- DOI: 10.1016/S2055-6640(20)30057-1
Identification of isoform-selective hydroxamic acid derivatives that potently reactivate HIV from latency
Abstract
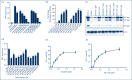
Objectives: Current antiretroviral therapy can suppress HIV replication, increase CD4 count and result in increased lifespan. However, it cannot eradicate the virus due to the presence of latent provirus in cellular reservoirs, such as resting CD4+ T cells. Using combination latency-reversing agents to shock the virus out of latency for elimination through immune clearance or viral cytopathic effect is one of the most promising strategies for HIV eradication. Specifically, recent evidence shows that isoform-selective histone deacetylase inhibitors may be more effective than their non-selective counterparts. Therefore, identification and characterisation of new isoform-selective compounds are of prime importance. Here, we sought to determine the ability of two new isoform-targeted hydroxamic acid derivatives to reactivate HIV from latency.
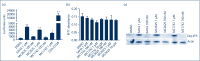
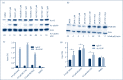
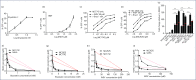
Methods: We used cell lines and infected primary resting CD4+ T cells. These were treated with these compounds with HIV reactivation measured using fluorescence-activated cell sorting, Western blots and luciferase luminescence. Isoform selectivity and acetylation of the HIV promoter were measured by Western blotting and chromatic immunoprecipitation.
Results and conclusions: The two new hydroxamic acid derivatives, MC2625 and MC1742, potently reactivate HIV from latency. These compounds are isoform-selective histone deacetylate inhibitors that increase the levels of histone acetylation at the HIV promoter. In addition, they synergise effectively with the protein kinase C modulators bryostatin-1 and INDY, an inhibitor of the dual-specificity tyrosine phosphorylation regulated kinase 1A. We conclude that the combinations of new hydroxamic acid derivatives and bryostatin-1 or INDY could be a new tool for HIV reactivation in the cure efforts.
Keywords: HIV; histone deacetylase inhibitors; isoform-targeted; latency.
Figures

Similar articles
-
Isoform-Selective Versus Nonselective Histone Deacetylase Inhibitors in HIV Latency Reversal.AIDS Res Hum Retroviruses. 2022 Aug;38(8):615-621. doi: 10.1089/AID.2021.0195. AIDS Res Hum Retroviruses. 2022. PMID: 35778852 Free PMC article. Review.
-
Quiescence Promotes Latent HIV Infection and Resistance to Reactivation from Latency with Histone Deacetylase Inhibitors.J Virol. 2017 Nov 30;91(24):e01080-17. doi: 10.1128/JVI.01080-17. Print 2017 Dec 15. J Virol. 2017. PMID: 29021396 Free PMC article.
-
Class 1-Selective Histone Deacetylase (HDAC) Inhibitors Enhance HIV Latency Reversal while Preserving the Activity of HDAC Isoforms Necessary for Maximal HIV Gene Expression.J Virol. 2018 Feb 26;92(6):e02110-17. doi: 10.1128/JVI.02110-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29298886 Free PMC article.
-
Alternate NF-κB-Independent Signaling Reactivation of Latent HIV-1 Provirus.J Virol. 2019 Aug 28;93(18):e00495-19. doi: 10.1128/JVI.00495-19. Print 2019 Sep 15. J Virol. 2019. PMID: 31243131 Free PMC article.
-
Reactivation of latent HIV by histone deacetylase inhibitors.Trends Microbiol. 2013 Jun;21(6):277-85. doi: 10.1016/j.tim.2013.02.005. Epub 2013 Mar 18. Trends Microbiol. 2013. PMID: 23517573 Free PMC article. Review.
Cited by
-
Isoform-Selective Versus Nonselective Histone Deacetylase Inhibitors in HIV Latency Reversal.AIDS Res Hum Retroviruses. 2022 Aug;38(8):615-621. doi: 10.1089/AID.2021.0195. AIDS Res Hum Retroviruses. 2022. PMID: 35778852 Free PMC article. Review.
-
An Amazing 30-Year Journey around the DABO Family: A Medicinal Chemistry Lesson on a Versatile Class of Non-nucleoside HIV-1 Reverse Transcriptase Inhibitors.J Med Chem. 2025 Mar 27;68(6):5993-6026. doi: 10.1021/acs.jmedchem.4c02848. Epub 2025 Mar 7. J Med Chem. 2025. PMID: 40053382 Free PMC article. Review.
-
Mini-review: Elucidating the psychological, physical, and sex-based interactions between HIV infection and stress.Neurosci Lett. 2021 Mar 16;747:135698. doi: 10.1016/j.neulet.2021.135698. Epub 2021 Feb 1. Neurosci Lett. 2021. PMID: 33540057 Free PMC article. Review.
-
The Role of Macrophages in HIV-1 Persistence and Pathogenesis.Front Microbiol. 2019 Dec 5;10:2828. doi: 10.3389/fmicb.2019.02828. eCollection 2019. Front Microbiol. 2019. PMID: 31866988 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
