In Situ Hybridisation Study of Neuronal Neuropeptides Expression in Models of Mandibular Denervation with or without Inflammation: Injury Dependant Neuropeptide Plasticity
- PMID: 31192032
- PMCID: PMC6561781
- DOI: 10.4172/2157-7099.1000509
In Situ Hybridisation Study of Neuronal Neuropeptides Expression in Models of Mandibular Denervation with or without Inflammation: Injury Dependant Neuropeptide Plasticity
Abstract
Neuronal expression of neuropeptides is altered following peripheral tissue injury associated with inflammation or nerve injury. This results in neuropathic pain with or without neurogenic inflammation which is a major health problem regularly seen in trigeminal neuralgia. Activation of the trigeminal system results in the release of vasoactive neuropeptides substance P and Calcitonin Gene-related Peptide (CGRP) which contribute to nociception, pain and neurogenic inflammation in injured tissues.
Aim: To study the alterations in the neuronal neuropeptides expressions in models of tissue injury associated with either nerve injury or with inflammation and to determine if denervation would alter the neuronal response to inflammation.
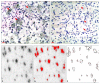
Material and methods: Experiments were performed on rat mandibles to produce three models. Firstly, denervation model by sectioning one of the mandibular nerve branches (inferior alveolar nerve). Secondarily, inflammation model by intra-gingival injection of lipopolysaccharide (LPS). Thirdly, combined denervation and inflammation model by sectioning the nerve with subsequent LPS injection. The animals were sacrificed seven days postoperative. Trigeminal ganglia on the operated sides were processed for in situ hybridisation for neuropeptides; substance P and CGRP mRNAs. Images were analysed for morphological and morphometric analysis using Image J software.
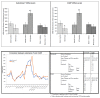
Results: substance P and CGRP mRNAs were expressed in small and medium-size primary afferent neurons in the mandibular division of the trigeminal ganglia. Both the denervation and the inflammation models showed alteration in neuropeptides expression in the sensory primary afferent neurons innervating the affected mandibular tissues. While, denervation resulted in a significant (substance P=P<0.04, CGRP=P<0.01) downregulation contrarily, inflammation resulted in a significant (P<0.001) upregulation of neuropeptides' mRNAs. Interestingly, denervation prior to induction of inflammation resulted in insignificant changes in neuropeptides levels. There was a strong correlation (Pearson Correlation=0.8) between substance P and CGRP expression.
Conclusion: We show that tissue damage associated with nerve injury or inflammation results in alteration of neuropeptides levels in the innervating primary afferent neurons. Tissue destruction associated with chronic inflammatory condition such as arthritis and periodontitis are believed to be due to the production of neuromodulators causing neurogenic inflammation. Here we show that denervation abolishes the neuronal response to inflammation. Therefore, tissues denervation could relieve neurogenic inflammation associated with chronic disorders through regulation of neuronal neuropeptide production. Moreover, the current model that combined denervation and inflammation provides a useful animal model to study the contribution of nerve-related mediators in the pathophysiology of tissue injury.
Keywords: Denervation; Inflammation; Neurogenic inflammation; Neuropeptides; Periodontitis.
Figures


Similar articles
-
Sensory neuropeptide mRNA up-regulation is bilateral in periodontitis in the rat: a possible neurogenic component to symmetrical periodontal disease.Eur J Neurosci. 2004 Feb;19(3):650-8. doi: 10.1111/j.1460-9568.2004.03179.x. Eur J Neurosci. 2004. PMID: 14984415
-
Haemodynamic and immunohistochemical studies of rat incisor pulp after denervation and subsequent re-innervation.Arch Oral Biol. 1995 Sep;40(9):815-23. doi: 10.1016/0003-9969(95)00048-t. Arch Oral Biol. 1995. PMID: 8651885
-
Deep tissue inflammation upregulates neuropeptides and evokes nociceptive behaviors which are modulated by a neuropeptide antagonist.Pain. 2006 Jan;120(1-2):53-68. doi: 10.1016/j.pain.2005.10.003. Epub 2005 Dec 13. Pain. 2006. PMID: 16359792
-
Current understanding of trigeminal ganglion structure and function in headache.Cephalalgia. 2019 Nov;39(13):1661-1674. doi: 10.1177/0333102418786261. Epub 2018 Jul 10. Cephalalgia. 2019. PMID: 29989427 Free PMC article. Review.
-
Neuropeptide effects in the trigeminal system: pathophysiology and clinical relevance in migraine.Keio J Med. 2011;60(3):82-9. doi: 10.2302/kjm.60.82. Keio J Med. 2011. PMID: 21979827 Review.
Cited by
-
Rapid and Controllable Multilayer Cell Sheet Assembly via Biodegradable Nanochannel Membranes.Adv Funct Mater. 2025 Jan 2;35(1):2403367. doi: 10.1002/adfm.202403367. Epub 2024 Jul 24. Adv Funct Mater. 2025. PMID: 40808793 Free PMC article.
-
Effect of Qinbai Qingfei Concentrated Pellets on substance P and neutral endopeptidase of rats with post-infectious cough.BMC Complement Med Ther. 2020 Sep 22;20(1):289. doi: 10.1186/s12906-020-03081-5. BMC Complement Med Ther. 2020. PMID: 32962697 Free PMC article.
References
-
- Lazarov NE. Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus. Prog Neurobiol. 2002;66:19–59. - PubMed
-
- Skofitsch G, Jacobowitz DM. Calcitonin gene-related peptide coexists with substance P in capsaicin-sensitive neurons and sensory ganglia of the rat. Peptides. 1985;6:747–754. - PubMed
-
- Reuss S, Riemann R, Vollrath L. Substance P and calcitonin gene-related peptide-like immunoreactive neurons in the rat trigeminal ganglion-with special reference to meningeal and pineal innervation. Acta Histochem. 1992;92:104–109. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
