Precipitating Factors and Treatment Outcomes of Hepatic Encephalopathy in Liver Cirrhosis
- PMID: 31192068
- PMCID: PMC6550494
- DOI: 10.7759/cureus.4363
Precipitating Factors and Treatment Outcomes of Hepatic Encephalopathy in Liver Cirrhosis
Abstract
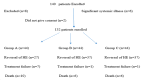
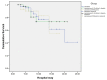
Background Hepatic encephalopathy (HE) is a common cause of hospital admission in patients with liver cirrhosis (LC). The aims of this study were to evaluate the precipitant factors and analyze the treatment outcomes of HE in LC. Methods All the LC patients admitted between February 2017 and January 2018 for overt HE were analyzed for precipitating factors and treatment outcomes. Treatments were compared among three treatment groups: receiving lactulose, lactulose plus L-ornithine L-aspartate (LOLA), and lactulose plus rifaximin. The primary endpoints were mortality and hospital stay. The chi-square test was used to compare the different treatment outcomes with hospital stay and mortality with significance at p<0.05. Results A total of 132 patients (mean age 49.2 ± 10.2 years; male/female ratio of 103:29) were studied. The most common precipitating factor of HE was infection 65 (49.2%), followed by electrolyte imbalance 54 (41%), constipation 44 (33.33%), and gastrointestinal bleeding 21 (16%) patients. At the time of admission, 29 (22%), 76 (57.5%), 21 (16%), and six (4.5%) patients had grade I, II, III, and IV HE, respectively. The difference in mortality was not statistically significant (p=0.269) in three groups but the hospital stay was shorter among patients in groups B and C than in group A alone (7.36 ± 4.58 and 7 ± 3.69, 9.64 ± 5.28 days, respectively, p=0.015). Conclusions Infection, especially spontaneous bacterial peritonitis, was the commonest precipitating factor of HE. The combination of lactulose either with LOLA or rifaximin is equally effective in improving HE and reducing the duration of hospital stay than lactulose alone.
Keywords: hepatic encephalopathy; liver cirrhosis; precipitating factors; treatment outcome.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Fitz JG. Gastrointestinal and Liver Disease. Philadelphia: Elsevier; 2016. Hepatic encephalopathy, hepatorenal syndrome, hepatopulmonary syndrome and other systemic complications of liver; pp. 1577–1590.
-
- Prognostic significance of hepatic encephalopathy in patients with cirrhosis. Bustamante J, Rimola A, Ventura PJ, Navasa M, Cirera I, Reggiardo V. https://www.sciencedirect.com/science/article/abs/pii/S0168827899801445. J Hepatol. 1999;30:890–895. - PubMed
-
- Hepatic encephalopathy—definition nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatology. 2002;35:716–721. - PubMed
-
- Blei AT, Bircher J, Benhamou JP, McIntyre N, Rizzetto M, Rodés J. Oxford Textbook of Clinical Hepatology. Oxford: Oxford Medical; 1998. Hepatic encephalopathy; pp. 765–783.
-
- Precipitating factors and the outcome of hepatic encephalopathy in liver cirrhosis. Mumtaz K, Ahmed US, Abid S, Baig N, Hamid S, Jafri W. https://www.ncbi.nlm.nih.gov/pubmed/20688015. J Coll Physicians Surg Pak. 2010;20:514–518. - PubMed
LinkOut - more resources
Full Text Sources