Characterization of the in vitro, ex vivo, and in vivo Efficacy of the Antimicrobial Peptide DPK-060 Used for Topical Treatment
- PMID: 31192163
- PMCID: PMC6548878
- DOI: 10.3389/fcimb.2019.00174
Characterization of the in vitro, ex vivo, and in vivo Efficacy of the Antimicrobial Peptide DPK-060 Used for Topical Treatment
Abstract
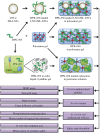
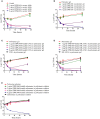
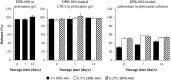
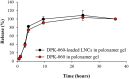
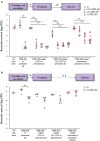
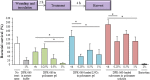
Antimicrobial peptides, also known as host defense peptides, have recently emerged as a promising new category of therapeutic agents for the treatment of infectious diseases. This study evaluated the preclinical in vitro, ex vivo, and in vivo antimicrobial activity, as well as the potential to cause skin irritation, of human kininogen-derived antimicrobial peptide DPK-060 in different formulations designed for topical delivery. We found that DPK-060 formulated in acetate buffer or poloxamer gel caused a marked reduction of bacterial counts of Staphylococcus aureus in vitro (minimum microbicidal concentration <5 μg/ml). We also found that DPK-060 in poloxamer gel significantly suppressed microbial survival in an ex vivo wound infection model using pig skin and in an in vivo mouse model of surgical site infection (≥99 or ≥94% reduction in bacterial counts was achieved with 1% DPK-060 at 4 h post-treatment, respectively). Encapsulation of DPK-060 in different types of lipid nanocapsules or cubosomes did not improve the bactericidal potential of the peptide under the applied test conditions. No reduction in cell viability was observed in response to administration of DPK-060 in any of the formulations tested. In conclusion, the present study confirms that DPK-060 has the potential to be an effective and safe drug candidate for the topical treatment of microbial infections; however, adsorption of the peptide to nanocarriers failed to show any additional benefits.
Keywords: DPK-060; antimicrobial peptides; cubosomes; lipid nanocapsules; skin infections.
Figures
Similar articles
-
Dendritic Nanogels Directed Dual-Encapsulation Topical Delivery System of Antimicrobial Peptides Targeting Skin Infections.Macromol Biosci. 2023 Apr;23(4):e2200433. doi: 10.1002/mabi.202200433. Epub 2023 Jan 22. Macromol Biosci. 2023. PMID: 36639138
-
Antimicrobial synergy of monolaurin lipid nanocapsules with adsorbed antimicrobial peptides against Staphylococcus aureus biofilms in vitro is absent in vivo.J Control Release. 2019 Jan 10;293:73-83. doi: 10.1016/j.jconrel.2018.11.018. Epub 2018 Nov 19. J Control Release. 2019. PMID: 30465823
-
Cubosomes for topical delivery of the antimicrobial peptide LL-37.Eur J Pharm Biopharm. 2019 Jan;134:60-67. doi: 10.1016/j.ejpb.2018.11.009. Epub 2018 Nov 13. Eur J Pharm Biopharm. 2019. PMID: 30445164
-
Antimicrobial peptides: potential use in skin infections.Am J Clin Dermatol. 2003;4(9):591-5. doi: 10.2165/00128071-200304090-00001. Am J Clin Dermatol. 2003. PMID: 12926977 Review.
-
Staphylococcal resistance to antimicrobial peptides of mammalian and bacterial origin.Peptides. 2001 Oct;22(10):1651-9. doi: 10.1016/s0196-9781(01)00500-9. Peptides. 2001. PMID: 11587793 Review.
Cited by
-
Shaping the Future of Antimicrobial Therapy: Harnessing the Power of Antimicrobial Peptides in Biomedical Applications.J Funct Biomater. 2023 Nov 2;14(11):539. doi: 10.3390/jfb14110539. J Funct Biomater. 2023. PMID: 37998108 Free PMC article. Review.
-
Lights and Shadows on the Therapeutic Use of Antimicrobial Peptides.Molecules. 2022 Jul 18;27(14):4584. doi: 10.3390/molecules27144584. Molecules. 2022. PMID: 35889455 Free PMC article. Review.
-
Biochemistry, Mechanistic Intricacies, and Therapeutic Potential of Antimicrobial Peptides: An Alternative to Traditional Antibiotics.Curr Med Chem. 2024;31(37):6110-6139. doi: 10.2174/0109298673268458230926105224. Curr Med Chem. 2024. PMID: 37818561 Review.
-
Are Antimicrobial Peptides a 21st-Century Solution for Atopic Dermatitis?Int J Mol Sci. 2023 Aug 30;24(17):13460. doi: 10.3390/ijms241713460. Int J Mol Sci. 2023. PMID: 37686269 Free PMC article. Review.
-
Clinical relevance of topical antibiotic use in co-selecting for multidrug-resistant Staphylococcus aureus: Insights from in vitro and ex vivo models.Antimicrob Agents Chemother. 2023 May 1;95(5):e02048-20. doi: 10.1128/AAC.02048-20. Epub 2021 Feb 16. Antimicrob Agents Chemother. 2023. PMID: 33593834 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

