Diverse Papillomavirus Types Induce Endosomal Tubulation
- PMID: 31192164
- PMCID: PMC6546808
- DOI: 10.3389/fcimb.2019.00175
Diverse Papillomavirus Types Induce Endosomal Tubulation
Abstract
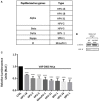
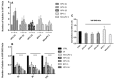
Previous studies have shown that the endoplasmic reticulum (ER)-anchored protein VAP is strictly required by human papillomavirus type 16 (HPV-16) for successful infectious entry. Entry appeared to be mediated in part through the induction of endosomal tubulation and subsequent transport of the virion to the trans-Golgi network (TGN). In this study, we were interested in investigating whether this mechanism of infectious entry is conserved across multiple Papillomavirus types. To do this, we analyzed the role of VAP and endosomal tubulation following infection with Pseudovirions (PsVs) derived from the alpha, beta, delta, kappa, and pi papillomavirus genera, reflecting viruses that are important human and animal pathogens. We demonstrate that VAP is essential for infection with all PV types analyzed. Furthermore, we find that VAP and EGFR-dependent endosomal tubulation is also induced by all these different Papillomaviruses. These results indicate an evolutionarily conserved requirement for VAP-induced endocytic tubulation during Papillomavirus infectious entry.
Keywords: MICAL-L1; PV trafficking; endosomal tubulation; infectious entry; papillomavirus.
Figures


Similar articles
-
Human Papillomavirus 16 Infection Induces VAP-Dependent Endosomal Tubulation.J Virol. 2018 Feb 26;92(6):e01514-17. doi: 10.1128/JVI.01514-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29321327 Free PMC article.
-
Rab6a enables BICD2/dynein-mediated trafficking of human papillomavirus from the trans-Golgi network during virus entry.mBio. 2024 Nov 13;15(11):e0281124. doi: 10.1128/mbio.02811-24. Epub 2024 Oct 21. mBio. 2024. PMID: 39431827 Free PMC article.
-
Phosphorylation of Human Papillomavirus Type 16 L2 Contributes to Efficient Virus Infectious Entry.J Virol. 2019 Jun 14;93(13):e00128-19. doi: 10.1128/JVI.00128-19. Print 2019 Jul 1. J Virol. 2019. PMID: 30996086 Free PMC article.
-
Cruising the cellular highways: How human papillomavirus travels from the surface to the nucleus.Virus Res. 2017 Mar 2;231:1-9. doi: 10.1016/j.virusres.2016.10.015. Epub 2016 Oct 29. Virus Res. 2017. PMID: 27984059 Free PMC article. Review.
-
Papillomaviruses and Endocytic Trafficking.Int J Mol Sci. 2018 Sep 4;19(9):2619. doi: 10.3390/ijms19092619. Int J Mol Sci. 2018. PMID: 30181457 Free PMC article. Review.
Cited by
-
Unraveling Immunological Dynamics: HPV Infection in Women-Insights from Pregnancy.Viruses. 2023 Sep 27;15(10):2011. doi: 10.3390/v15102011. Viruses. 2023. PMID: 37896788 Free PMC article. Review.
-
A peptide derived from sorting nexin 1 inhibits HPV16 entry, retrograde trafficking, and L2 membrane spanning.Tumour Virus Res. 2024 Dec;18:200287. doi: 10.1016/j.tvr.2024.200287. Epub 2024 Jun 21. Tumour Virus Res. 2024. PMID: 38909779 Free PMC article.
-
Human Papillomavirus infection requires the CCT Chaperonin Complex.J Virol. 2021 May 10;95(11):e01943-20. doi: 10.1128/JVI.01943-20. Epub 2021 Mar 17. J Virol. 2021. PMID: 33731457 Free PMC article.
-
Human Papillomavirus 16 L2 Recruits both Retromer and Retriever Complexes during Retrograde Trafficking of the Viral Genome to the Cell Nucleus.J Virol. 2021 Jan 13;95(3):e02068-20. doi: 10.1128/JVI.02068-20. Print 2021 Jan 13. J Virol. 2021. PMID: 33177206 Free PMC article.
-
Recent Advances in Our Understanding of the Infectious Entry Pathway of Human Papillomavirus Type 16.Microorganisms. 2021 Oct 1;9(10):2076. doi: 10.3390/microorganisms9102076. Microorganisms. 2021. PMID: 34683397 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

