Risk Factors for Indeterminate Interferon-Gamma Release Assay for the Diagnosis of Tuberculosis in Children-A Systematic Review and Meta-Analysis
- PMID: 31192175
- PMCID: PMC6548884
- DOI: 10.3389/fped.2019.00208
Risk Factors for Indeterminate Interferon-Gamma Release Assay for the Diagnosis of Tuberculosis in Children-A Systematic Review and Meta-Analysis
Abstract
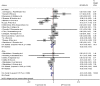
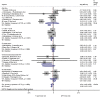
Background: Interferon-gamma release assays (IGRA) are well-established immunodiagnostic tests for tuberculosis (TB) in adults. In children these tests are associated with higher rates of false-negative and indeterminate results. Age is presumed to be one factor influencing cytokine release and therefore test performance. The aim of this study was to systematically review factors associated with indeterminate IGRA results in pediatric patients. Methods: Systematic literature review guided by the preferred reporting items for systematic reviews and meta-analyses (PRISMA) searching PubMed, EMBASE, and Web of Science. Studies reporting results of at least one commercially available IGRA (QuantiFERON-TB, T-SPOT.TB) in pediatric patient groups were included. Random effects meta-analysis was used to assess proportions of indeterminate IGRA results. Heterogeneity was assessed using the I2 value. Risk differences were calculated for studies comparing QuantiFERON-TB and T-SPOT.TB in the same study. Meta-regression was used to further explore the influence of study level variables on heterogeneity. Results: Of 1,293 articles screened, 133 studies were included in the final analysis. These assessed QuantiFERON-TB only in 77.4% (103/133), QuantiFERON-TB and T-SPOT.TB in 15.8% (21/133), and T-SPOT.TB only in 6.8% (9/133) resulting in 155 datasets including 107,418 participants. Overall 4% of IGRA results were indeterminate, and T-SPOT.TB (0.03, 95% CI 0.02-0.05) and QuantiFERON-TB assays (0.05, 95% CI 0.04-0.06) showed similar proportions of indeterminate results; pooled risk difference was-0.01 (95% CI -0.03 to 0.00). Significant differences with lower proportions of indeterminate assays with T-SPOT.TB compared to QuantiFERON-TB were only seen in subgroup analyses of studies performed in Africa and in non-HIV-infected immunocompromised patients. Meta-regression confirmed lower proportions of indeterminate results for T-SPOT.TB compared to QuantiFERON-TB only among studies that reported results from non-HIV-infected immunocompromised patients (p < 0.001). Conclusion: On average indeterminate IGRA results occur in 1 in 25 tests performed. Overall, there was no difference in the proportion of indeterminate results between both commercial assays. However, our findings suggest that in patients in Africa and/or patients with immunocompromising conditions other than HIV infection the T-SPOT.TB assay appears to produce fewer indeterminate results.
Keywords: Clinical studies; IGRA; QuantiFERON; T cell response; T-SPOT.TB; latent; pediatrics; risk difference.
Figures




Similar articles
-
Indeterminate results of interferon gamma release assays in the screening of latent tuberculosis infection: a systematic review and meta-analysis.Front Immunol. 2023 May 15;14:1170579. doi: 10.3389/fimmu.2023.1170579. eCollection 2023. Front Immunol. 2023. PMID: 37256138 Free PMC article.
-
Prevalence of indeterminate tuberculosis interferon-gamma release assays in COVID-19 patients: Systematic review and meta-analysis.Health Sci Rep. 2023 Dec 20;6(12):e1695. doi: 10.1002/hsr2.1695. eCollection 2023 Dec. Health Sci Rep. 2023. PMID: 38130328 Free PMC article.
-
Performance of commercial blood tests for the diagnosis of latent tuberculosis infection in children and adolescents.Pediatrics. 2009 Mar;123(3):e419-24. doi: 10.1542/peds.2008-1722. Pediatrics. 2009. PMID: 19254978
-
Diagnosis of Tuberculous Infection in Immunosuppressed Patients and/or Candidates for Biologics Using a Combination of 2 IGRA Tests: T-SPOT.TB/QuantiFERON TB Gold In-Tube vs. T-SPOT.TB/QuantiFERON TB Gold Plus.Arch Bronconeumol. 2022 Apr;58(4):305-310. doi: 10.1016/j.arbres.2020.04.011. Epub 2020 Jun 10. Arch Bronconeumol. 2022. PMID: 32534870 English, Spanish.
-
Interferon-γ release assays for the diagnosis of Mycobacterium tuberculosis infection in children: a literature review.Int J Immunopathol Pharmacol. 2012 Apr-Jun;25(2):335-43. doi: 10.1177/039463201202500203. Int J Immunopathol Pharmacol. 2012. PMID: 22697065 Review.
Cited by
-
COVID-19 and Tuberculosis: Mathematical Modeling of Infection Spread Taking into Account Reduced Screening.Diagnostics (Basel). 2024 Mar 26;14(7):698. doi: 10.3390/diagnostics14070698. Diagnostics (Basel). 2024. PMID: 38611611 Free PMC article.
-
THE ROLE OF INTERFERON-GAMMA RELEASE ASSAYS IN DIAGNOSIS OF LATENT TUBERCULOSIS INFECTION IN CHILDREN.Acta Clin Croat. 2023 Nov;62(3):527-538. doi: 10.20471/acc.2023.62.03.15. Acta Clin Croat. 2023. PMID: 39310695 Free PMC article. Review.
-
A data driven policy to minimise the tuberculosis testing cost among healthcare workers.Int J Health Plann Manage. 2022 Sep;37(5):2697-2709. doi: 10.1002/hpm.3496. Epub 2022 May 8. Int J Health Plann Manage. 2022. PMID: 35527355 Free PMC article.
-
Performance evaluation of the LIOFeron®TB/LTBI IGRA for screening of paediatric LTBI and tuberculosis.Eur J Pediatr. 2025 Jan 20;184(2):147. doi: 10.1007/s00431-025-05972-6. Eur J Pediatr. 2025. PMID: 39831989 Free PMC article.
-
Preliminary evaluation of a new prototype interferon-gamma release assay for the detection of Mycobacterium tuberculosis-specific T-cell responses in patients with tuberculosis.Folia Microbiol (Praha). 2025 Apr;70(2):493-503. doi: 10.1007/s12223-025-01252-w. Epub 2025 Mar 3. Folia Microbiol (Praha). 2025. PMID: 40032785 Free PMC article.
References
-
- World Health Organization Global Tuberculosis Report 2018 (2018).
Publication types
LinkOut - more resources
Full Text Sources

