Complex Neurological Phenotype in Female Carriers of NHE6 Mutations
- PMID: 31192222
- PMCID: PMC6528080
- DOI: 10.1159/000496341
Complex Neurological Phenotype in Female Carriers of NHE6 Mutations
Abstract
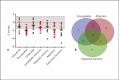
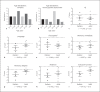
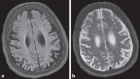
Mutations in NHE6 (also termed SLC9A6) cause the X-linked neurological disorder Christianson syndrome (CS) in males. The purpose of this study was to examine the phenotypic spectrum of female carriers of NHE6 mutations. Twenty female carriers from 9 pedigrees were enrolled, ranging from approximately age 2 to 65. A subset of female carriers was assessed using standardized neuropsychological measures. Also, the association of NHE6 expression with markers of brain age was evaluated using 740 participants in the Religious Orders Study (ROS) and Rush Memory and Aging Project (MAP). A majority, but not all, female carriers demonstrated a deficit in at least one neurocognitive domain (85%). A recognizable neuropsychological profile emerged, revealing impairments in visuospatial function, attention, and executive function. Common neuropsychiatric diagnoses included: intellectual disability/developmental delay (20%), learning difficulties (31%), speech/language delays (30%), and attention-deficit/hyperactivity disorder (20%). Notable neurological diagnoses in aging CS female carriers include corticobasal degeneration and atypical parkinsonism. In postmortem brains from the ROS/MAP dataset of normal and pathological aging, decreased NHE6 expression was correlated with greater tau deposition. Our study provides an examination of the phenotypic range in female carriers of NHE6 mutations. The findings indicate that NHE6-related disease in females represents a new neurogenetic condition.
Keywords: Christianson syndrome; Female carriers; Na+/H+ exchanger 6 (NHE6); X-chromosome inactivation (XCI).
Figures

References
-
- Christianson AL, Stevenson RE, van der Meyden CH, Pelser J, Theron FW, van Rensburg PL, et al. X linked severe mental retardation, craniofacial dysmorphology, epilepsy, ophthalmoplegia, and cerebellar atrophy in a large South African kindred is localised to Xq24-q27. J Med Genet. 1999 Oct;36((10)):759–66. - PMC - PubMed
-
- Mignot C, Héron D, Bursztyn J, Momtchilova M, Mayer M, Whalen S, et al. Novel mutation in SLC9A6 gene in a patient with Christianson syndrome and retinitis pigmentosum. Brain Dev. 2013 Feb;35((2)):172–6. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

