Inducible nitric oxide synthase: Regulation, structure, and inhibition
- PMID: 31192483
- PMCID: PMC6908786
- DOI: 10.1002/med.21599
Inducible nitric oxide synthase: Regulation, structure, and inhibition
Abstract
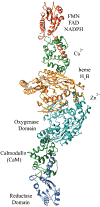
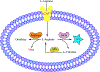
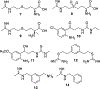
A considerable number of human diseases have an inflammatory component, and a key mediator of immune activation and inflammation is inducible nitric oxide synthase (iNOS), which produces nitric oxide (NO) from l-arginine. Overexpressed or dysregulated iNOS has been implicated in numerous pathologies including sepsis, cancer, neurodegeneration, and various types of pain. Extensive knowledge has been accumulated about the roles iNOS plays in different tissues and organs. Additionally, X-ray crystal and cryogenic electron microscopy structures have shed new insights on the structure and regulation of this enzyme. Many potent iNOS inhibitors with high selectivity over related NOS isoforms, neuronal NOS, and endothelial NOS, have been discovered, and these drugs have shown promise in animal models of endotoxemia, inflammatory and neuropathic pain, arthritis, and other disorders. A major issue in iNOS inhibitor development is that promising results in animal studies have not translated to humans; there are no iNOS inhibitors approved for human use. In addition to assay limitations, both the dual modalities of iNOS and NO in disease states (ie, protective vs harmful effects) and the different roles and localizations of NOS isoforms create challenges for therapeutic intervention. This review summarizes the structure, function, and regulation of iNOS, with focus on the development of iNOS inhibitors (historical and recent). A better understanding of iNOS' complex functions is necessary before specific drug candidates can be identified for classical indications such as sepsis, heart failure, and pain; however, newer promising indications for iNOS inhibition, such as depression, neurodegenerative disorders, and epilepsy, have been discovered.
Keywords: animal models; cancer; enzyme inhibition; immune regulation; immune system activation; inducible nitric oxide synthase; inflammation; neurodegeneration; nitrergic signaling; nitric oxide; pain; reactive oxygen species; sepsis.
© 2019 Wiley Periodicals, Inc.
Figures
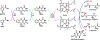


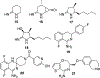
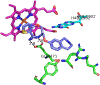
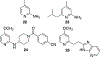


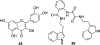
References
-
- Abu-Soud HM, Gachhui R, Raushel FM, et al. The ferrous-dioxy complex of neuronal nitric oxide synthase: divergent effects of l-arginine and tetrahydrobiopterin on its stability. J Biol Chem. 1997;272:17349–17353. - PubMed
-
- Kone BC, Kuncewicz T, Zhang W, et al. Protein interactions with nitric oxide synthases: controlling the right time, the right place, and the right amount of nitric oxide. Am J Physiol - Renal. 2003;285:F178–F190. - PubMed
-
- Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15:252–259. - PubMed
-
- MacMicking J, Xie Q-w, Nathan C Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

