Identification of infants with increased type 1 diabetes genetic risk for enrollment into Primary Prevention Trials-GPPAD-02 study design and first results
- PMID: 31192505
- PMCID: PMC6851563
- DOI: 10.1111/pedi.12870
Identification of infants with increased type 1 diabetes genetic risk for enrollment into Primary Prevention Trials-GPPAD-02 study design and first results
Abstract
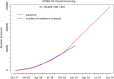
Primary prevention of type 1 diabetes (T1D) requires intervention in genetically at-risk infants. The Global Platform for the Prevention of Autoimmune Diabetes (GPPAD) has established a screening program, GPPAD-02, that identifies infants with a genetic high risk of T1D, enrolls these into primary prevention trials, and follows the children for beta-cell autoantibodies and diabetes. Genetic testing is offered either at delivery, together with the regular newborn testing, or at a newborn health care visits before the age of 5 months in regions of Germany (Bavaria, Saxony, Lower Saxony), UK (Oxford), Poland (Warsaw), Belgium (Leuven), and Sweden (Region Skåne). Seven clinical centers will screen around 330 000 infants. Using a genetic score based on 46 T1D susceptibility single-nucleotide polymorphisms (SNPs) or three SNPS and a first-degree family history for T1D, infants with a high (>10%) genetic risk for developing multiple beta-cell autoantibodies by the age of 6 years are identified. Screening from October 2017 to December 2018 was performed in 50 669 infants. The prevalence of high genetic risk for T1D in these infants was 1.1%. Infants with high genetic risk for T1D are followed up and offered to participate in a randomized controlled trial aiming to prevent beta-cell autoimmunity and T1D by tolerance induction with oral insulin. The GPPAD-02 study provides a unique path to primary prevention of beta-cell autoimmunity in the general population. The eventual benefit to the community, if successful, will be a reduction in the number of children developing beta-cell autoimmunity and T1D.
Keywords: beta-cell autoantibodies; genetic risk for type 1 diabetes; type 1 diabetes.
© 2019 The Authors. Pediatric Diabetes published by John Wiley & Sons Ltd.
Figures

References
-
- Patterson CC, Harjutsalo V, Rosenbauer J, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989‐2013: a multicentre prospective registration study. Diabetologia. 2019;62(3):408‐417. - PubMed
-
- Bonifacio E. Predicting type 1 diabetes using biomarkers. Diabetes Care. 2015;38(6):989‐996. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

