Low-Resistance, Concentric-Gated Pediatric Artificial Lung for End-Stage Lung Failure
- PMID: 31192843
- PMCID: PMC7293821
- DOI: 10.1097/MAT.0000000000001018
Low-Resistance, Concentric-Gated Pediatric Artificial Lung for End-Stage Lung Failure
Abstract
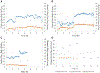
Children with end-stage lung failure awaiting lung transplant would benefit from improvements in artificial lung technology allowing for wearable pulmonary support as a bridge-to-transplant therapy. In this work, we designed, fabricated, and tested the Pediatric MLung-a dual-inlet hollow fiber artificial lung based on concentric gating, which has a rated flow of 1 L/min, and a pressure drop of 25 mm Hg at rated flow. This device and future iterations of the current design are designed to relieve pulmonary arterial hypertension, provide pulmonary support, reduce ventilator-associated injury, and allow for more effective therapy of patients with end-stage lung disease, including bridge-to-transplant treatment.
Conflict of interest statement
Disclosure: The authors have no conflicts of interest to report.
Figures





References
-
- Valapour M, Skeans MA, Smith JM, et al.: OPTN/SRTR 2015 annual data report: lung. Am J Transplant 17(suppl 1): 357–424, 2017. - PubMed
-
- Boston US, Fehr J, Gazit AZ, Eghtesady P: Paracorporeal lung assist device: an innovative surgical strategy for bridging to lung transplant in an infant with severe pulmonary hypertension caused by alveolar capillary dysplasia. J Thorac Cardiovasc Surg 146: e42–e43, 2013. - PubMed
-
- Hoganson DM, Gazit AZ, Boston US, et al.: Paracorporeal lung assist devices as a bridge to recovery or lung transplantation in neonates and young children. J Thorac Cardiovasc Surg 147: 420–426, 2014. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

