Oncologic outcomes of IMRT versus CRT for nasopharyngeal carcinoma: A meta-analysis
- PMID: 31192932
- PMCID: PMC6587629
- DOI: 10.1097/MD.0000000000015951
Oncologic outcomes of IMRT versus CRT for nasopharyngeal carcinoma: A meta-analysis
Abstract
Background: Nasopharyngeal carcinoma (NPC) is a prevalent malignancy in Asia particularly southern China. Comparisons of outcomes of conformal radiotherapy (CRT) and intensity-modulated radiotherapy (IMRT) have been still debated. This meta-analysis was carried out to compare oncologic outcomes of CRT and IMRT in the treatment of NPC.
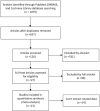
Methods: A literature search was performed through PubMed, Embase, and the Cochrane library databases from their inceptions to November 10, 2018. Two authors assessed the included studies and extracted data independently. Relative studies that compared oncologic outcomes between CRT and IMRT for NPC were included.
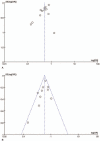
Results: A total of 13 eligible studies were included, which contained 1 RCT, 1 prospective study, and 11 retrospective studies. Our meta-analysis showed that IMRT has increased overall survival (OR = 0.51, 95% CI = 0.41-0.65, P < .00001), locoregional control rate (OR = 0.59, 95% CI = 0.52-0.67, P < .00001), disease-free survival (OR = 0.77, 95% CI = 0.65-0.91, P = .002), and metastasis-free survival (OR = 0.71, 95% CI = 0.54-0.94, P = .01) in comparison with CRT.
Conclusion: The results of this meta-analysis indicate IMRT should be a better option for the treatment of NPC because patients who underwent IMRT may benefit from increased overall survival, locoregional control rate, disease-free survival, and metastasis-free survival compared with CRT.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


Similar articles
-
Outcomes and prognostic factors of conformal radiotherapy versus intensity-modulated radiotherapy for nasopharyngeal carcinoma.Clin Transl Oncol. 2012 Oct;14(10):783-90. doi: 10.1007/s12094-012-0864-5. Epub 2012 Jul 24. Clin Transl Oncol. 2012. PMID: 22855156
-
Clinical outcome of intensity-modulated radiotherapy versus two-dimensional conventional radiotherapy in locally advanced nasopharyngeal carcinoma: Comparative study at SKIMS Tertiary Care Institute.J Cancer Res Ther. 2022 Jan-Mar;18(1):133-139. doi: 10.4103/jcrt.jcrt_169_21. J Cancer Res Ther. 2022. PMID: 35381774
-
IMRT vs. 2D-radiotherapy or 3D-conformal radiotherapy of nasopharyngeal carcinoma : Survival outcome in a Korean multi-institutional retrospective study (KROG 11-06).Strahlenther Onkol. 2016 Jun;192(6):377-85. doi: 10.1007/s00066-016-0959-y. Epub 2016 Mar 14. Strahlenther Onkol. 2016. PMID: 26972085 Review. English.
-
Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: A 10-year experience with a large cohort and long follow-up.Eur J Cancer. 2015 Nov;51(17):2587-95. doi: 10.1016/j.ejca.2015.08.006. Epub 2015 Aug 26. Eur J Cancer. 2015. PMID: 26318726
-
Long-term outcomes after reirradiation in nasopharyngeal carcinoma with intensity-modulated radiotherapy: A meta-analysis.Head Neck. 2018 Mar;40(3):622-631. doi: 10.1002/hed.24993. Epub 2017 Nov 11. Head Neck. 2018. PMID: 29130584 Review.
Cited by
-
Epigallocatechin-3-Gallate Protects Pro-Acinar Epithelia Against Salivary Gland Radiation Injury.Int J Mol Sci. 2021 Mar 19;22(6):3162. doi: 10.3390/ijms22063162. Int J Mol Sci. 2021. PMID: 33808935 Free PMC article.
-
The Chinese Society of Clinical Oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma.Cancer Commun (Lond). 2021 Nov;41(11):1195-1227. doi: 10.1002/cac2.12218. Epub 2021 Oct 26. Cancer Commun (Lond). 2021. PMID: 34699681 Free PMC article.
-
Distribution of regional lymph nodes metastasis in 870 cases of nasopharyngeal carcinoma and the suggestions for individualized elective prophylactic neck irradiation with intensity-modulated radiotherapy.Cancer Med. 2024 Feb;13(3):e6723. doi: 10.1002/cam4.6723. Epub 2023 Dec 29. Cancer Med. 2024. PMID: 38156901 Free PMC article.
-
Predicting the efficacy of chemoradiotherapy in advanced nasopharyngeal carcinoma patients: an MRI radiomics and machine learning approach.Front Oncol. 2025 Jun 26;15:1554899. doi: 10.3389/fonc.2025.1554899. eCollection 2025. Front Oncol. 2025. PMID: 40641917 Free PMC article.
-
Effect of radiotherapy interruption on nasopharyngeal cancer.Front Oncol. 2023 Apr 6;13:1114652. doi: 10.3389/fonc.2023.1114652. eCollection 2023. Front Oncol. 2023. PMID: 37091186 Free PMC article. Review.
References
-
- Chan OS, Sze HC, Lee MC, et al. Reirradiation with intensity-modulated radiotherapy for locally recurrent T3 to T4 nasopharyngeal carcinoma. Head Neck 2017;39:533–40. - PubMed
-
- Li JG, Venigalla P, Leeman JE, et al. Patterns of nodal failure after intensity modulated radiotherapy for nasopharyngeal carcinoma. Laryngoscope 2017;127:377–82. - PubMed
-
- Wu LR, Liu YT, Jiang N, et al. Ten-year survival outcomes for patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy: an analysis of 614 patients from a single center. Oral Oncol 2017;69:26–32. - PubMed
-
- Veldeman L, Madani I, Hulstaert F, et al. Evidence behind use of intensity-modulated radiotherapy: a systematic review of comparative clinical studies. Lancet Oncol 2008;9:367–75. - PubMed
-
- Qiu WZ, Peng XS, Xia HQ, et al. A retrospective study comparing the outcomes and toxicities of intensity-modulated radiotherapy versus two-dimensional conventional radiotherapy for the treatment of children and adolescent nasopharyngeal carcinoma. J Cancer Res Clin Oncol 2017;143:1563–72. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

