LW6 enhances chemosensitivity to gemcitabine and inhibits autophagic flux in pancreatic cancer
- PMID: 31193017
- PMCID: PMC6514270
- DOI: 10.1016/j.jare.2019.04.006
LW6 enhances chemosensitivity to gemcitabine and inhibits autophagic flux in pancreatic cancer
Abstract
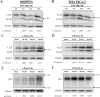
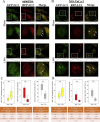
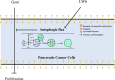
The efficacy of gemcitabine therapy is often insufficient for the treatment of pancreatic cancer. The current study demonstrated that LW6, a chemical inhibitor of hypoxia-inducible factor 1α, is a promising drug for enhancing the chemosensitivity to gemcitabine. LW6 monotherapy and the combination therapy of LW6 plus gemcitabine significantly inhibited cell proliferation and enhanced cell death in pancreatic cancer cells. This combination therapy also significantly reduced the tumor weight in a syngeneic orthotopic pancreatic carcinoma model without causing toxic side effects. In addition, this study provides insight into the mechanism of how LW6 interferes with the pathophysiology of pancreatic cancer. The results revealed that LW6 inhibited autophagic flux, which is defined by the accumulation of microtubule-associated protein 1 light chain 3 (LC3) and p62/SQSTM1. Moreover, these results were verified by the analysis of a tandem RFP-GFP-tagged LC3 protein. Thence, for the first time, these data demonstrate that LW6 enhances the anti-tumor effects of gemcitabine and inhibits autophagic flux. This suggests that the combination therapy of LW6 plus gemcitabine may be a novel therapeutic strategy for pancreatic cancer patients.
Keywords: 3-MA, 3-methyladenine; ATCC, American Type Culture Collection; Autophagy; BrdU, 5-bromo-2′-deoxyuridine; CQ, chloroquine; Combination therapy; Gemcitabine; LC3, microtubule-associated protein 1 light chain 3; LW6; Pancreatic cancer; p62, p62/SQSTM1.
Figures









References
-
- Hu X.C., Zhang J., Xu B.H., Cai L., Ragaz J., Wang Z.H. Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015;16:436–446. - PubMed
-
- Thatcher N., Hirsch F.R., Luft A.V., Szczesna A., Ciuleanu T.E., Dediu M. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16:763–774. - PubMed
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. - PubMed
-
- Lee K.1, Kang J.E., Park S.K., Jin Y., Chung K.S., Kim H.M. LW6, a novel HIF-1 inhibitor, promotes proteasomal degradation of HIF-1alpha via upregulation of VHL in a colon cancer cell line. Biochem Pharmacol. 2010;80:982–989. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

