Histological evaluation of tendon formation using a scaffold-free three-dimensional-bioprinted construct of human dermal fibroblasts under in vitro static tensile culture
- PMID: 31193148
- PMCID: PMC6517794
- DOI: 10.1016/j.reth.2019.02.002
Histological evaluation of tendon formation using a scaffold-free three-dimensional-bioprinted construct of human dermal fibroblasts under in vitro static tensile culture
Abstract
Introduction: Tendon tissue engineering requires scaffold-free techniques for safe and long-term clinical applications and to explore alternative cell sources to tenocytes. Therefore, we histologically assessed tendon formation in a scaffold-free Bio-three-dimensional (3D) construct developed from normal human dermal fibroblasts (NHDFs) using our Bio-3D printer system under tensile culture in vitro.
Methods: Scaffold-free ring-like tissues were constructed from 120 multicellular spheroids comprising NHDFs using a bio-3D printer. Ring-like tissues were cultured in vitro under static tensile-loading with or without in-house tensile devices (tension-loaded and tension-free groups), with increases in tensile strength applied weekly to the tensile-loaded group. After a 4 or 8-week culture on the device, we evaluated histological findings according to tendon-maturing score and immunohistological findings of the middle portion of the tissues for both groups (n = 4, respectively).
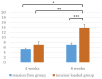
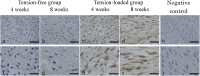
Results: Histology of the tension-loaded group revealed longitudinally aligned collagen fibers with increased collagen deposition and spindle-shaped cells with prolonged culture. By contrast, the tension-free group showed no organized cell arrangement or collagen fiber structure. Additionally, the tension-loaded group showed a significantly improved tendon-maturing score as compared with that for the tension-free group at week 8. Moreover, immunohistochemistry revealed tenascin C distribution with a parallel arrangement in the tensile-loading direction at week 8 in the tension-loaded group, which exhibited stronger scleraxis-staining intensity than that observed in the tension-free group at weeks 4 and 8.
Conclusions: The NHDF-generated scaffold-free Bio-3D construct underwent remodeling and formed tendon-like structures under tensile culture in vitro.
Keywords: 3D, three-dimensional; ECM, extracellular matrix; ESCs, embryonic stem cells; H&E, hematoxylin and eosin; HDF, human dermal fibroblast; Human dermal fibroblast; In vitro study; MCSs, multicellular spheroids; Multicellular spheroid; NHDFs, normal HDFs; Scaffold-free; TDSCs, tendon-derived stem cells; TGF, transforming growth factor; Tendon formation; Tensile culture.
Conflict of interest statement
K. Nakayama is a co-founder and shareholder of Cyfuse Biomedical KK and an investor/developer designated on the patent for the Bio-3D printer. Patent title: Method for Production of Three-Dimensional Structure of Cells; patent number: JP4517125. Patent title: Cell Structure Production Device; patent number: JP5896104.
Figures




Similar articles
-
[In vitro tendon engineering using human dermal fibroblasts].Zhonghua Yi Xue Za Zhi. 2008 Apr 1;88(13):914-8. Zhonghua Yi Xue Za Zhi. 2008. PMID: 18756959 Chinese.
-
Engineering human neo-tendon tissue in vitro with human dermal fibroblasts under static mechanical strain.Biomaterials. 2009 Dec;30(35):6724-30. doi: 10.1016/j.biomaterials.2009.08.054. Epub 2009 Sep 25. Biomaterials. 2009. PMID: 19782396
-
Repair of tendon defect with dermal fibroblast engineered tendon in a porcine model.Tissue Eng. 2006 Apr;12(4):775-88. doi: 10.1089/ten.2006.12.775. Tissue Eng. 2006. PMID: 16674291
-
Recent Advances in Three-Dimensional Multicellular Spheroid Culture and Future Development.Micromachines (Basel). 2021 Jan 18;12(1):96. doi: 10.3390/mi12010096. Micromachines (Basel). 2021. PMID: 33477508 Free PMC article. Review.
-
Scaffold-free cell-based tissue engineering therapies: advances, shortfalls and forecast.NPJ Regen Med. 2021 Mar 29;6(1):18. doi: 10.1038/s41536-021-00133-3. NPJ Regen Med. 2021. PMID: 33782415 Free PMC article. Review.
Cited by
-
Application of suture anchors for a clinically relevant rat model of rotator cuff tear.J Tissue Eng Regen Med. 2022 Aug;16(8):757-770. doi: 10.1002/term.3326. Epub 2022 Jun 7. J Tissue Eng Regen Med. 2022. PMID: 35670621 Free PMC article.
-
Bio 3D Conduits Derived from Bone Marrow Stromal Cells Promote Peripheral Nerve Regeneration.Cell Transplant. 2020 Jan-Dec;29:963689720951551. doi: 10.1177/0963689720951551. Cell Transplant. 2020. PMID: 32830545 Free PMC article.
-
3D cell aggregate printing technology and its applications.Essays Biochem. 2021 Aug 10;65(3):467-480. doi: 10.1042/EBC20200128. Essays Biochem. 2021. PMID: 34223609 Free PMC article. Review.
-
Rebuilding Tendons: A Concise Review on the Potential of Dermal Fibroblasts.Cells. 2020 Sep 8;9(9):2047. doi: 10.3390/cells9092047. Cells. 2020. PMID: 32911760 Free PMC article. Review.
-
Macromolecular crowding in human tenocyte and skin fibroblast cultures: A comparative analysis.Mater Today Bio. 2024 Jan 28;25:100977. doi: 10.1016/j.mtbio.2024.100977. eCollection 2024 Apr. Mater Today Bio. 2024. PMID: 38322661 Free PMC article.
References
-
- Huang H.H., Qureshi A.A., Biundo J.J. Sports and other soft tissue injuries, tendinitis, bursitis, and occupation-related syndromes. Curr Opin Rheumatol. 2000;12:150–154. - PubMed
-
- Chen J.M., Xu J.K., Wang A.L., Zheng M.H. Scaffolds for tendon and ligament repair: review of the efficacy of commercial products. Expert Rev Med Devices. 2009;6:61–73. - PubMed
-
- Yamamoto A., Takagishi K., Osawa T., Yanagawa T., Nakajima D., Shitara H. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010;19:116–120. - PubMed
-
- Clayton R.A.E., Court-Brown C.M. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury. 2008;39:1338–1344. - PubMed
-
- Cerullo G., Puddu G., Gianní E., Damiani A., Pigozzi F. Anterior cruciate ligament patellar tendon reconstruction: it is probably better to leave the tendon defect open! Knee Surg Sports Traumatol Arthrosc. 1995;3(1):14–17. - PubMed

