Characterization of immune response in Cross-Reactive Immunological Material (CRIM)-positive infantile Pompe disease patients treated with enzyme replacement therapy
- PMID: 31193175
- PMCID: PMC6518314
- DOI: 10.1016/j.ymgmr.2019.100475
Characterization of immune response in Cross-Reactive Immunological Material (CRIM)-positive infantile Pompe disease patients treated with enzyme replacement therapy
Abstract
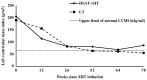
Enzyme replacement therapy (ERT) with rhGAA has improved clinical outcomes in infantile Pompe disease (IPD). A subset of CRIM-positive IPD patients develop high and sustained antibody titers (HSAT; ≥51,200) and/or sustained intermediate titer (SIT; ≥12,800 and <51,200), similar to CRIM-negative patients. To date there has been no systematic study to analyze the extent of IgG antibody response in CRIM-positive IPD. Such data would be critical and could serve as a comparator group for potential immune modulation approaches. A retrospective analysis of the dataset from the original rhGAA clinical trials final reports was conducted. CRIM-positive patients who received ERT monotherapy and had >6 months of antibody titer data available, were included in the study. Patients were classified based on their longitudinal antibody titers into HSAT, SIT, and low titer (LT; <12,800) groups. Of the 37 patients that met inclusion criteria, five (13%), seven (19%), and 25 (68%) developed HSAT, SIT, and LT, respectively. Median peak titers were 204,800 (51,200-409,600), 25,600 (12,800-51,200), and 800 (200-12,800) for HSAT, SIT, and LT groups, respectively. Median last titers were 102,400 (51,200-409,600), 1600 (200-25,600), and 400 (0-12,800) at median time since ERT initiation of 94 weeks (64-155 weeks), 104 weeks (86-144 weeks), and 130 weeks (38-182 weeks) for HSAT, SIT, and LT groups, respectively. 32% (12/37) of CRIM-positive IPD patients developed HSAT/SIT which may lead to limited ERT response and clinical decline. Further Studies are needed to identify CRIM-positive IPD patients at risk of developing HSAT/SIT, especially with the addition of Pompe disease to the newborn screening.
Keywords: AIMS, Alberta infant motor scale; Anti-rhGAA Ig antibodies; Antidrug antibodies; CI-MPR, Cation-independent mannose 6-phosphate receptor; CRIM, Cross-reactive immunological material; EOW, Every other week; ERT, Enzyme replacement therapy; Enzyme replacement therapy; GAA, Acid α-glucosidase; GAA, Gene encoding acid α-glucosidase; Glc4, Glucose tetrasaccharide; Glycogen storage disease type II; HLA, Human leukocyte antigen; HSAT, High and sustained antibody titers; IPD, Infantile Pompe disease; IgG, Immunoglobulin G; LT, Low titers; LVMI, Left ventricular mass index; MHC, Major histocompatibility complex; Neuromuscular disease; Pompe disease; RUSP, Recommended universal screening panel; SIT, Sustained intermediate titers; iTEM, Individualized T-cell epitope measure; rhGAA, Recombinant human acid α-glucosidase.
Figures
Similar articles
-
CRIM-negative infantile Pompe disease: characterization of immune responses in patients treated with ERT monotherapy.Genet Med. 2015 Nov;17(11):912-8. doi: 10.1038/gim.2015.6. Epub 2015 Mar 5. Genet Med. 2015. PMID: 25741864 Free PMC article.
-
High dose IVIG successfully reduces high rhGAA IgG antibody titers in a CRIM-negative infantile Pompe disease patient.Mol Genet Metab. 2017 Sep;122(1-2):76-79. doi: 10.1016/j.ymgme.2017.05.006. Epub 2017 May 18. Mol Genet Metab. 2017. PMID: 28648664 Free PMC article.
-
An immune tolerance approach using transient low-dose methotrexate in the ERT-naïve setting of patients treated with a therapeutic protein: experience in infantile-onset Pompe disease.Genet Med. 2019 Apr;21(4):887-895. doi: 10.1038/s41436-018-0270-7. Epub 2018 Sep 14. Genet Med. 2019. PMID: 30214072 Free PMC article.
-
Immunological challenges and approaches to immunomodulation in Pompe disease: a literature review.Ann Transl Med. 2019 Jul;7(13):285. doi: 10.21037/atm.2019.05.27. Ann Transl Med. 2019. PMID: 31392197 Free PMC article. Review.
-
Higher dosing of alglucosidase alfa improves outcomes in children with Pompe disease: a clinical study and review of the literature.Genet Med. 2020 May;22(5):898-907. doi: 10.1038/s41436-019-0738-0. Epub 2020 Jan 6. Genet Med. 2020. PMID: 31904026 Free PMC article. Review.
Cited by
-
HLA- and genotype-based risk assessment model to identify infantile onset pompe disease patients at high-risk of developing significant anti-drug antibodies (ADA).Clin Immunol. 2019 Mar;200:66-70. doi: 10.1016/j.clim.2019.01.009. Epub 2019 Jan 31. Clin Immunol. 2019. PMID: 30711607 Free PMC article.
-
Optimizing treatment outcomes: immune tolerance induction in Pompe disease patients undergoing enzyme replacement therapy.Front Immunol. 2024 Apr 23;15:1336599. doi: 10.3389/fimmu.2024.1336599. eCollection 2024. Front Immunol. 2024. PMID: 38715621 Free PMC article. Clinical Trial.
-
Are Anti-rhGAA Antibodies a Determinant of Treatment Outcome in Adults with Late-Onset Pompe Disease? A Systematic Review.Biomolecules. 2023 Sep 19;13(9):1414. doi: 10.3390/biom13091414. Biomolecules. 2023. PMID: 37759814 Free PMC article.
-
Severe CNS involvement in a subset of long-term treated children with infantile-onset Pompe disease.Mol Genet Metab. 2024 Feb;141(2):108119. doi: 10.1016/j.ymgme.2023.108119. Epub 2023 Dec 22. Mol Genet Metab. 2024. PMID: 38184429 Free PMC article.
-
Phenotypic implications of pathogenic variant types in Pompe disease.J Hum Genet. 2021 Nov;66(11):1089-1099. doi: 10.1038/s10038-021-00935-9. Epub 2021 May 11. J Hum Genet. 2021. PMID: 33972680
References
-
- Amalfitano A., Bengur A.R., Morse R.P., Majure J.M., Case L.E., Veerling D.L., Mackey J., Kishnani P., Smith W., McVie-Wylie A., Sullivan J.A., Hoganson G.E., Phillips J.A., III, Schaefer G.B., Charrow J., Ware R.E., Bossen E.H., Chen Y.T. Recombinant human acid alpha-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial. Genet Med. 2001;3(2):132–138. - PubMed
-
- Bali D.S., Goldstein J.L., Banugaria S., Dai J., Mackey J., Rehder C., Kishnani P.S. Predicting cross-reactive immunological material (CRIM) status in Pompe disease using GAA mutations: lessons learned from 10 years of clinical laboratory testing experience. Am. J. Med. Genet. C: Semin. Med. Genet. 2012;160C(1):40–49. - PMC - PubMed
-
- Banugaria S.G., Prater S.N., Ng Y.K., Kobori J.A., Finkel R.S., Ladda R.L., Chen Y.T., Rosenberg A.S., Kishnani P.S. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease. Genet Med. 2011;13(8):729–736. - PMC - PubMed
-
- Berrier K.L., Kazi Z.B., Prater S.N., Bali D.S., Goldstein J., Stefanescu M.C., Rehder C.W., Botha E.G., Ellaway C., Bhattacharya K., Tylki-Szymanska A., Karabul N., Rosenberg A.S., Kishnani P.S. CRIM-negative infantile Pompe disease: characterization of immune responses in patients treated with ERT monotherapy. Genet Med. 2015;17(11):912–918. - PMC - PubMed
-
- De Filippi P., Saeidi K., Ravaglia S., Dardis A., Angelini C., Mongini T., Morandi L., Moggio M., Di Muzio A., Filosto M., Bembi B., Giannini F., Marrosu G., Rigoldi M., Tonin P., Servidei S., Siciliano G., Carlucci A., Scotti C., Comelli M., Toscano A., Danesino C. Genotype-phenotype correlation in Pompe disease, a step forward. Orphanet J Rare Dis. 2014;9:102. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

