Beneficial role of bioactive lipids in the pathobiology, prevention, and management of HBV, HCV and alcoholic hepatitis, NAFLD, and liver cirrhosis: A review
- PMID: 31193303
- PMCID: PMC6526165
- DOI: 10.1016/j.jare.2018.12.006
Beneficial role of bioactive lipids in the pathobiology, prevention, and management of HBV, HCV and alcoholic hepatitis, NAFLD, and liver cirrhosis: A review
Abstract
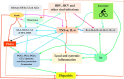
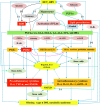
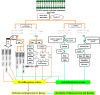
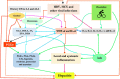
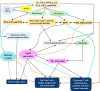
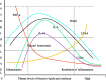
It has been suggested that hepatitis B virus (HBV)- and hepatitis C virus (HCV)-induced hepatic damage and cirrhosis and associated hypoalbuminemia, non-alcoholic fatty liver disease (NAFLD), and alcoholic fatty liver disease (AFLD) are due to an imbalance between pro-inflammatory and anti-inflammatory bioactive lipids. Increased tumour necrosis factor (TNF)-α production induced by HBV and HCV leads to a polyunsaturated fatty acid (PUFA) deficiency and hypoalbuminemia. Albumin mobilizes PUFAs from the liver and other tissues and thus may aid in enhancing the formation of anti-inflammatory lipoxins, resolvins, protectins, maresins and prostaglandin E1 (PGE1) and suppressing the production of pro-inflammatory PGE2. As PUFAs exert anti-viral and anti-bacterial effects, the presence of adequate levels of PUFAs could inactivate HCV and HBV and prevent spontaneous bacterial peritonitis observed in cirrhosis. PUFAs, PGE1, lipoxins, resolvins, protectins, and maresins suppress TNF-α and other pro-inflammatory cytokines, exert cytoprotective effects, and modulate stem cell proliferation and differentiation to promote recovery following hepatitis, NAFLD and AFLD. Based on this evidence, it is proposed that the administration of albumin in conjunction with PUFAs and their anti-inflammatory products could be beneficial for the prevention of and recovery from NAFLD, hepatitis and cirrhosis of the liver. NAFLD is common in obesity, type 2 diabetes mellitus, and metabolic syndrome, suggesting that even these diseases could be due to alterations in the metabolism of PUFAs and other bioactive lipids. Hence, PUFAs and co-factors needed for their metabolism and albumin may be of benefit in the prevention and management of HBV, HCV, alcoholic hepatitis and NAFLD, and liver cirrhosis.
Keywords: Cirrhosis; Cytokines; Hepatitis; Non-alcoholic fatty liver disease; Polyunsaturated fatty acids.
Figures
References
-
- Perz J.F., Armstrong G.L., Farrington L.A., Hutin Y.J., Bell B.P. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. - PubMed
-
- Kim E.H., Bae J.S., Hahm K.B., Cha J.Y. Endogenously synthesized n-3 polyunsaturated fatty acids in fat-1 mice ameliorate high-fat diet-induced non-alcoholic fatty liver disease. Biochem Pharmacol. 2012;84:1359–1365. - PubMed
-
- Fernández I., Torres I., Moreira E., Fontana L., Gil A., Rios A. Influence of administration of long-chain polyunsaturated fatty acids on process of histological recovery in liver cirrhosis produced by oral intake of thioacetamide. Dig Dis Sci. 1996;41:197–207. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

