Enrichment factors for clinical trials in mild-to-moderate Alzheimer's disease
- PMID: 31193334
- PMCID: PMC6527908
- DOI: 10.1016/j.trci.2019.04.001
Enrichment factors for clinical trials in mild-to-moderate Alzheimer's disease
Abstract
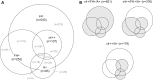
Introduction: Heterogeneity of outcomes in Alzheimer's disease (AD) clinical trials necessitates large sample sizes and contributes to study failures. This analysis determined whether mild-to-moderate AD populations could be enriched for cognitive decline based on apolipoprotein (APOE) ε4 genotype, family history of AD, and amyloid abnormalities.
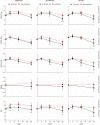
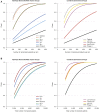
Methods: Modeling estimated the number of randomized patients needed to detect a 2-point treatment difference on the AD Assessment Scale-Cognitive subscale using placebo data from three randomized, double-blind trials (ClinicalTrials.gov Identifiers: NCT01955161, NCT02006641, and NCT02006654).
Results: An 80% power to detect a 2-point treatment effect required the randomization of 148 amyloid-positive patients; 178 ε4 homozygous or amyloid-positive patients; and 231 ε4 homozygous, family history-positive, or amyloid-positive patients, compared with 1619 unenriched patients (per arm).
Discussion: Enrichment in mild-to-moderate AD clinical trials can be achieved using combinations of biomarkers/risk factors to increase the likelihood of observing potential treatment effects.
Keywords: Alzheimer's disease; Biomarkers; Clinical trial; Enrichment; Power.
Figures

References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
