Characterization and densification of defect pyrochlore oxide powders ABi2Ta5O16 (A=Na, Tl)
- PMID: 31193356
- PMCID: PMC6525300
- DOI: 10.1016/j.heliyon.2019.e01628
Characterization and densification of defect pyrochlore oxide powders ABi2Ta5O16 (A=Na, Tl)
Erratum in
-
Erratum to "Characterization and densification of defect pyrochlore oxide powders ABi2Ta5O16 (A=Na, Tl)" [Heliyon 5 (5) (May 2019) e01628].Heliyon. 2019 Jun 21;5(6):e01891. doi: 10.1016/j.heliyon.2019.e01891. eCollection 2019 Jun. Heliyon. 2019. PMID: 31294095 Free PMC article.
Abstract
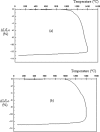
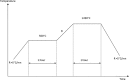
Defect pyrochlore oxide powders ABi2Ta5O16, in the pseudo-binary systems BiTaO4-ATa3O8 (A = Na, Tl), were prepared by solid state method. The structural study showed that all oxides crystallize in a cubic system with the space group Fd3m, the lattice parameter "a" were determined using Rietveld refinement method. For systematic study on densification from powders, the samples were pressed with a uniaxial pressure into pellets; sintering temperature, holding time and heating rate were optimized. Techniques, including X-ray diffraction, IR-Raman, MEB, dilatometry, were employed to investigate the structure and the morphology of the synthesized powders and sintered materials. The dielectric characteristics, relative permittivity and dielectric losses (tgδ), determined at room temperature are comparable to those of other pyrochlores.
Keywords: Inorganic chemistry; Materials chemistry.
Figures









Similar articles
-
Erratum to "Characterization and densification of defect pyrochlore oxide powders ABi2Ta5O16 (A=Na, Tl)" [Heliyon 5 (5) (May 2019) e01628].Heliyon. 2019 Jun 21;5(6):e01891. doi: 10.1016/j.heliyon.2019.e01891. eCollection 2019 Jun. Heliyon. 2019. PMID: 31294095 Free PMC article.
-
Pyrochlore structure and spectroscopic studies of titanate ceramics. A comparative investigation on SmDyTi2O7 and YDyTi2O7 solid solutions.Spectrochim Acta A Mol Biomol Spectrosc. 2018 Jun 5;198:188-197. doi: 10.1016/j.saa.2018.03.024. Epub 2018 Mar 10. Spectrochim Acta A Mol Biomol Spectrosc. 2018. PMID: 29547820
-
Os4+ Instability in the Pyrochlore Structure: Tl2-xBixOs2O7-y.Inorg Chem. 2020 Jan 21;59(2):1227-1233. doi: 10.1021/acs.inorgchem.9b02939. Epub 2020 Jan 7. Inorg Chem. 2020. PMID: 31909983
-
Cu, Mg Codoped Bismuth Tantalate Pyrochlores: Crystal Structure, XPS Spectra, Thermal Expansion, and Electrical Properties.Inorg Chem. 2022 Mar 14;61(10):4270-4282. doi: 10.1021/acs.inorgchem.1c03053. Epub 2022 Mar 3. Inorg Chem. 2022. PMID: 35239334
-
Ceramic Mineral Waste-Forms for Nuclear Waste Immobilization.Materials (Basel). 2019 Aug 19;12(16):2638. doi: 10.3390/ma12162638. Materials (Basel). 2019. PMID: 31430956 Free PMC article. Review.
Cited by
-
Toward Efficient Electrocatalytic Oxygen Evolution: Emerging Opportunities with Metallic Pyrochlore Oxides for Electrocatalysts and Conductive Supports.ACS Cent Sci. 2020 Jun 24;6(6):880-891. doi: 10.1021/acscentsci.0c00479. Epub 2020 Jun 5. ACS Cent Sci. 2020. PMID: 32607435 Free PMC article. Review.
References
-
- Subramanian M.A., Aravamudan G., SubbaRao G.V. Oxide pyrochlores -A review. Prog. Solid State Chem. 1983;15:55–143.
-
- Yonezawa S., Muraoka Y., Matsushita Y., Hiroi Superconductivity in a pyrochlore-related oxide KOs2O6. J. Phys. Condens. Matter. 2004;16:9–12.
-
- Sudheendran K., Raju K.C.J., Jacob M.V. Microwave Dielectric Properties of Ti-Substituted Bi2 (Zn2/3Nb4/3)O7 Pyrochlores at Cryogenic Temperatures. J. Am. Ceram. Soc. 2009;92(6):1268–1271.
-
- Youn H.J., Sogabe T., Randall C.A., Shrout T.R., T M. Phase Relations and Dielectric Properties in the Bi2O3–ZnO–Ta2O5 System. J. Am. Ceram. Soc. 2001;84:2557–2562.
-
- Khaw C.C., Tan K.B., Lee C.K. High temperature dielectric properties of cubic bismuth zinc tantalate. Ceram. Int. 2009;35:1473–1480.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

