Pre-clinical study protocol: Blood transfusion in endotoxaemic shock
- PMID: 31193460
- PMCID: PMC6529713
- DOI: 10.1016/j.mex.2019.05.005
Pre-clinical study protocol: Blood transfusion in endotoxaemic shock
Abstract
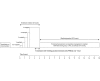
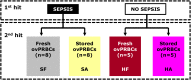
The Surviving Sepsis Campaign (SCC) and the American College of Critical Care Medicine (ACCM) guidelines recommend blood transfusion in sepsis when the haemoglobin concentration drops below 7.0 g/dL and 10.0 g/dL respectively, while the World Health Organisation (WHO) guideline recommends transfusion in septic shock 'if intravenous (IV) fluids do not maintain adequate circulation', as a supportive measure of last resort. Volume expansion using crystalloid and colloid fluid boluses for haemodynamic resuscitation in severe illness/sepsis, has been associated with adverse outcomes in recent literature. However, the volume expansion effect(s) following blood transfusion for haemodynamic circulatory support, in severe illness remain unclear with most previous studies having focused on evaluating effects of either different RBC storage durations (short versus long duration) or haemoglobin thresholds (low versus high threshold) pre-transfusion. •We describe the protocol for a pre-clinical randomised controlled trial designed to examine haemodynamic effect(s) of early volume expansion using packed RBCs (PRBCs) transfusion (before any crystalloids or colloids) in a validated ovine-model of hyperdynamic endotoxaemic shock.•Additional exploration of mechanisms underlying any physiological, haemodynamic, haematological, immunologic and tissue specific-effects of blood transfusion will be undertaken including comparison of effects of short (≤5 days) versus long (≥30 days) storage duration of PRBCs prior to transfusion.
Keywords: Blood transfusion; Endotoxaemic shock; Guidelines; Haemoglobin threshold; Packed red blood cells (PRBCs); Sepsis; Storage duration.
Figures
References
-
- Dellinger R.P., Levy M.M., Rhodes A., Annane D., Gerlach H., Opal S.M., Sevransky J.E., Sprung C.L., Douglas I.S., Jaeschke R. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013;41(2):580–637. - PubMed
-
- Rhodes A., Evans L.E., Alhazzani W., Levy M.M., Antonelli M., Ferrer R., Kumar A., Sevransky J.E., Sprung C.L., Nunnally M.E. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. - PubMed
-
- Davis A.L., Carcillo J.A., Aneja R.K., Deymann A.J., Lin J.C., Nguyen T.C., Okhuysen-Cawley R.S., Relvas M.S., Rozenfeld R.A., Skippen P.W. American college of critical care medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit. Care Med. 2017;45(6):1061–1093. - PubMed
-
- Karam O., Tucci M., Ducruet T., Hume H.A., Lacroix J., Gauvin F., Canadian Critical Care Trials G, Network P Red blood cell transfusion thresholds in pediatric patients with sepsis. Pediatr. Crit. Care Med. 2011;12(5):512–518. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials