Synthesis and characterization of Zn-Doped hydroxyapatite: scaffold application, antibacterial and bioactivity studies
- PMID: 31193510
- PMCID: PMC6531666
- DOI: 10.1016/j.heliyon.2019.e01716
Synthesis and characterization of Zn-Doped hydroxyapatite: scaffold application, antibacterial and bioactivity studies
Abstract
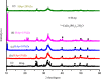
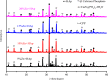
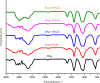
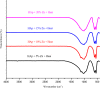
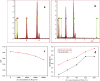
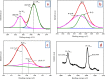
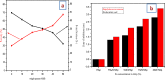
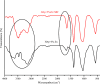
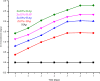
In this study, the antimicrobial and scaffold of zinc-substituted hydroxyapatite, (Zn-HAp) synthesized via chemical co-precipitation technique was investigated. The structure of the synthesized Zn-HAp was investigated with X-ray diffraction (XRD), Fourier transform infrared (FT-IR) spectroscopy, Scanning electron microscope (SEM), Energy dispersive X-spectroscopy (EDAX), transmission electron microscope (TEM), Thermogravimetric analysis (TGA) and X-ray photoelectron spectroscopy (XPS). Bioactivity study was performed in simulated body fluid (SBF), while the antimicrobial activity was studied using disc diffusion method. The XRD structure revealed that Zn ion incorporation up to 10% led to the second phase hydroxyapatite (HAp) formation, while higher concentration diminished the apatite structure. The presence of phosphate ions, carbonates ions, and hydroxyl groups in the apatite powder was ascertained by the FT-IR evaluation. SEM evaluation showed that the apatite contains fine particles with nearly round shape with interconnected pores and decreasing Ca/P ratio with increasing Zn ion concentration. TEM results showed particulate polycrystalline apatite with crystallite size ranging from 68 nm in pure HAp to 41 nm in 20% Zn-doped HAp indicating a decrease in the crystal size with increasing Zn ion in the samples. The bioactivity study showed spherical deposition around the porous region of the scaffold HAp suggesting the growth of apatite in SBF media after 7 days of incubation, while antibacterial activity studies showed zones of inhibition with an increase in zinc ions concentrations.
Keywords: Materials chemistry; Materials science.
Figures
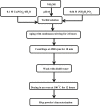





References
-
- Jarcho M. Retrospective analysis of hydroxyapatite development for oral implant applications. Dent. Clin. N. Am. 1992;36:19–26. - PubMed
-
- Fathi M.H., Hanifi A. Evaluation and characterization of nanostructure hydroxyapatite powder prepared by simple sol–gel method. Mater. Lett. 2007;61:3978–3983.
-
- Ramakrishnan R., Wilson P., Sivakumar T., Jemina I. A. comparative study of hydroxyapatites synthesized using various fuels through aqueous and alcohol mediated combustion routes. Ceram. Int. 2013;39:3519–3532.
-
- Webster T.J., Massa-Schlueter E.A., Smith J.L., Slamovich E.B. Osteoblast response to hydroxyapatite doped with divalent and trivalent cations. Biomaterials. 2004;25:2111–2121. - PubMed
-
- Kaygili O., Tatar C. The investigation of some physical properties and microstructure of Zn-doped hydroxyapatite bioceramics prepared by sol–gel method. J. Sol. Gel Sci. Technol. 2012;61:296–309.
LinkOut - more resources
Full Text Sources
Miscellaneous

