Age- and sex-dependent impact of repeated social stress on morphology of rat prefrontal cortex pyramidal neurons
- PMID: 31193524
- PMCID: PMC6535647
- DOI: 10.1016/j.ynstr.2019.100165
Age- and sex-dependent impact of repeated social stress on morphology of rat prefrontal cortex pyramidal neurons
Abstract
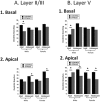
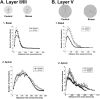
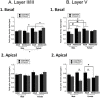
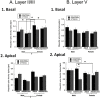
Chronic stress can lead to psychiatric illness characterized by impairments of executive function, implicating the prefrontal cortex as a target of stress-related pathology. Previous studies have shown that various types of chronic stress paradigms reduce dendritic branching, length and spines of medial prefrontal cortex (mPFC) pyramidal neurons. However, these studies largely focused on layer II/III pyramidal neurons in adult male rats with less known about layer V, the site of projection neurons. Because the prefrontal cortex develops throughout adolescence, stress during adolescence may have a greater impact on structure and function than stress occurring during adulthood. Furthermore, females display greater risk of stress-related psychiatric disorders, indicating sex-specific responses to stress. In this study, male and female adolescent (42-48 days old, 4 rats per group) or adult (68-72 days old, 4 rats per group) Sprague-Dawley rats were exposed to 5 days of repeated social stress in the resident-intruder paradigm or control manipulation. We examined dendritic morphology of cells in the mPFC in both layer II/III and Layer V. Repeated social stress resulted in decreased dendritic branching in layer II/III apical dendrites regardless of sex or age. In apical layer V dendrites, stress increased branching in adult males but decreased it in all other groups. Stress resulted in a decrease in dendritic spines in layer V apical dendrites for male adolescents and female adults, and this was mostly due to a decrease in filopodial and mushroom spines for male adolescents, but stubby spines for female adults. In sum, these results suggest that repeated stress reduces complexity and synaptic connectivity in adolescents and female adults in both input and output layers of prelimbic mPFC, but not in male adults. These changes may represent a potential underlying mechanism as to why adolescents and females are more susceptible to the negative cognitive effects of repeated or chronic stress.
Keywords: Development; Golgi stain; Morphology; Prefrontal cortex; Sex; Stress.
Figures
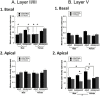
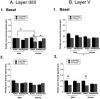
Similar articles
-
Age- and Sex-Dependent Impact of Repeated Social Stress on Intrinsic and Synaptic Excitability of the Rat Prefrontal Cortex.Cereb Cortex. 2017 Jan 1;27(1):244-253. doi: 10.1093/cercor/bhw388. Cereb Cortex. 2017. PMID: 28013234 Free PMC article.
-
Differential dendritic remodeling in prelimbic cortex of male and female rats during recovery from chronic stress.Neuroscience. 2017 Aug 15;357:145-159. doi: 10.1016/j.neuroscience.2017.05.049. Epub 2017 Jun 9. Neuroscience. 2017. PMID: 28596115 Free PMC article.
-
Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration.J Neurobiol. 2001 Nov 15;49(3):245-53. doi: 10.1002/neu.1079. J Neurobiol. 2001. PMID: 11745662
-
Stress-induced alterations in prefrontal dendritic spines: Implications for post-traumatic stress disorder.Neurosci Lett. 2015 Aug 5;601:41-5. doi: 10.1016/j.neulet.2014.12.035. Epub 2014 Dec 18. Neurosci Lett. 2015. PMID: 25529195 Review.
-
Plasticity in the prefrontal cortex of adult rats.Front Cell Neurosci. 2015 Feb 3;9:15. doi: 10.3389/fncel.2015.00015. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25691857 Free PMC article. Review.
Cited by
-
In search of sex-related mediators of affective illness.Biol Sex Differ. 2021 Oct 18;12(1):55. doi: 10.1186/s13293-021-00400-4. Biol Sex Differ. 2021. PMID: 34663459 Free PMC article. Review.
-
Adolescent Alcohol and Stress Exposure Rewires Key Cortical Neurocircuitry.Front Neurosci. 2022 May 17;16:896880. doi: 10.3389/fnins.2022.896880. eCollection 2022. Front Neurosci. 2022. PMID: 35655755 Free PMC article.
-
Sex-Specific Role for SLIT1 in Regulating Stress Susceptibility.Biol Psychiatry. 2022 Jan 1;91(1):81-91. doi: 10.1016/j.biopsych.2021.01.019. Epub 2021 Feb 27. Biol Psychiatry. 2022. PMID: 33896623 Free PMC article.
-
Influences of age and pubertal status on number and intensity of perineuronal nets in the rat medial prefrontal cortex.Brain Struct Funct. 2020 Nov;225(8):2495-2507. doi: 10.1007/s00429-020-02137-z. Epub 2020 Sep 10. Brain Struct Funct. 2020. PMID: 32914251 Free PMC article.
-
Integrative Brain Dynamics in Childhood Bullying Victimization: Cognitive and Emotional Convergence Associated With Stress Psychopathology.Front Integr Neurosci. 2022 Apr 27;16:782154. doi: 10.3389/fnint.2022.782154. eCollection 2022. Front Integr Neurosci. 2022. PMID: 35573445 Free PMC article. Review.
References
-
- Adler N.E., Boyce T., Chesney M.A., Cohen S., Folkman S., Kahn R.L., Syme S.L. Socioeconomic status and health. The challenge of the gradient. Am. Psychol. 1994;49:15–24. - PubMed
-
- Bangasser D.A., Curtis A., Reyes B.A., Bethea T.T., Parastatidis I., Ischiropoulos H., Van Bockstaele E.J., Valentino R.J. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatr. 2010;15(877):896–904. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

