Interventions after acute stress prevent its delayed effects on the amygdala
- PMID: 31193585
- PMCID: PMC6535648
- DOI: 10.1016/j.ynstr.2019.100168
Interventions after acute stress prevent its delayed effects on the amygdala
Abstract
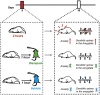
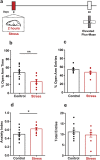
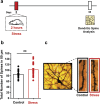
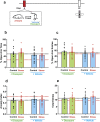
Stress is known to elicit contrasting patterns of plasticity in the amygdala and hippocampus. While chronic stress leads to neuronal atrophy in the rodent hippocampus, it has the opposite effect in the basolateral amygdala (BLA). Further, even a single episode of acute stress is known to elicit delayed effects in the amygdala. For example, 2 h of immobilisation stress has been shown to cause a delayed increase in dendritic spine density on BLA principal neurons 10 days later in young rats. This is paralleled by higher anxiety-like behaviour at the same delayed time point. This temporal build-up of morphological and behavioural effects 10 days later, in turn, provides a stress-free time window of intervention after exposure to acute stress. Here, we explore this possibility by specifically testing the efficacy of an anxiolytic drug in reversing the delayed effects of acute immobilisation stress. Oral gavage of diazepam 1 h after immobilisation stress prevented the increase in anxiety-like behaviour on the elevated plus-maze 10 days later. The same post-stress intervention also prevented delayed spinogenesis in the BLA 10 days after acute stress. Surprisingly, gavage of only the vehicle also had a protective effect on both the behavioural and synaptic effects of stress 10 days later. Vehicle gavage was found to trigger a significant rise in corticosterone levels that was comparable to that elicited by acute stress. This suggests that a surge in corticosterone levels, caused by the vehicle gavage 1 h after acute stress, was capable of reversing the delayed enhancing effects of stress on anxiety-like behaviour and BLA synaptic connectivity. These findings are consistent with clinical reports on the protective effects of glucocorticoids against the development of symptoms of post-traumatic stress disorder. Taken together, these results reveal strategies, targeted 1 h after stress, which can prevent the delayed effects of a brief exposure to a severe physical stressor.
Keywords: Anxiety; Basolateral amygdala; Corticosterone; Dendritic spines; Diazepam; Stress.
Figures

Similar articles
-
Corticosterone after acute stress prevents the delayed effects on the amygdala.Neuropsychopharmacology. 2020 Dec;45(13):2139-2146. doi: 10.1038/s41386-020-0758-0. Epub 2020 Jul 6. Neuropsychopharmacology. 2020. PMID: 32629457 Free PMC article.
-
Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala.Biol Psychiatry. 2012 Sep 15;72(6):466-75. doi: 10.1016/j.biopsych.2012.04.008. Epub 2012 May 8. Biol Psychiatry. 2012. PMID: 22572034 Free PMC article.
-
The delayed strengthening of synaptic connectivity in the amygdala depends on NMDA receptor activation during acute stress.Physiol Rep. 2016 Oct;4(20):e13002. doi: 10.14814/phy2.13002. Physiol Rep. 2016. PMID: 27798355 Free PMC article.
-
Role of the basolateral amygdala in memory consolidation.Prog Neurobiol. 2003 Aug;70(5):409-20. doi: 10.1016/s0301-0082(03)00104-7. Prog Neurobiol. 2003. PMID: 14511699 Review.
-
Stress as a one-armed bandit: Differential effects of stress paradigms on the morphology, neurochemistry and behavior in the rodent amygdala.Neurobiol Stress. 2015 Jun 9;1:195-208. doi: 10.1016/j.ynstr.2015.06.001. eCollection 2015 Jan. Neurobiol Stress. 2015. PMID: 26844236 Free PMC article. Review.
Cited by
-
Corticosterone after acute stress prevents the delayed effects on the amygdala.Neuropsychopharmacology. 2020 Dec;45(13):2139-2146. doi: 10.1038/s41386-020-0758-0. Epub 2020 Jul 6. Neuropsychopharmacology. 2020. PMID: 32629457 Free PMC article.
-
Medial prefrontal cortex input to basolateral amygdala controls acute stress-induced short-term anxiety-like behavior in mice.Neuropsychopharmacology. 2023 Apr;48(5):734-744. doi: 10.1038/s41386-022-01515-x. Epub 2022 Dec 13. Neuropsychopharmacology. 2023. PMID: 36513871 Free PMC article.
-
Transient Elevation of Plasma Glucocorticoids Supports Psilocybin-Induced Anxiolysis in Mice.ACS Pharmacol Transl Sci. 2023 Aug 2;6(8):1221-1231. doi: 10.1021/acsptsci.3c00123. eCollection 2023 Aug 11. ACS Pharmacol Transl Sci. 2023. PMID: 37588757 Free PMC article.
-
Administration of the glutamate-modulating drug, riluzole, after stress prevents its delayed effects on the amygdala in male rats.PNAS Nexus. 2023 May 22;2(6):pgad166. doi: 10.1093/pnasnexus/pgad166. eCollection 2023 Jun. PNAS Nexus. 2023. PMID: 37266396 Free PMC article.
-
PPM1F in Dentate Gyrus Modulates Anxiety-Related Behaviors by Regulating BDNF Expression via AKT/JNK/p-H3S10 Pathway.Mol Neurobiol. 2021 Jul;58(7):3529-3544. doi: 10.1007/s12035-021-02340-x. Epub 2021 Mar 21. Mol Neurobiol. 2021. PMID: 33745117
References
-
- Brown A.P., Dinger N., Levine B.S. Stress produced by gavage administration in the rat. Contemp. Top. Lab. Anim. Sci. 2000;39:17–21. - PubMed
LinkOut - more resources
Full Text Sources

