Systematic De-escalation of Successful Triple Antiretroviral Therapy to Dual Therapy with Dolutegravir plus Emtricitabine or Lamivudine in Swiss HIV-positive Persons
- PMID: 31193647
- PMCID: PMC6537552
- DOI: 10.1016/j.eclinm.2018.11.005
Systematic De-escalation of Successful Triple Antiretroviral Therapy to Dual Therapy with Dolutegravir plus Emtricitabine or Lamivudine in Swiss HIV-positive Persons
Abstract
Background: Studies increasingly suggest that the efficacy of certain dual antiretroviral therapy (ART) combinations is equal to triple ART. Increasing concerns among HIV-positive patients and physicians in Switzerland include ART cost and long-term ART safety and toxicity, i.e. taking only as many ART agents as necessary. The aims of this retrospective analysis are to report on the de-escalation of our entire clinic population of eligible patients with well-controlled HIV-infection to dolutegravir-containing dual ART.
Methods: Starting in March 2015, we systematically considered the de-escalation of eligible patients to either dolutegravir/emtricitabine or dolutegravir/lamivudine, by discontinuing tenofovir disoproxil fumarate or abacavir. We report on the virological efficacy, tolerability and patient satisfaction ≥ 48 weeks after de-escalation.
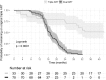
Findings: Of 106 HIV-positive patients followed in our clinic, 70 patients were de-escalated. Three returned to triple ART (insomnia after dolutegravir start, n = 2; new wish for single tablet regimen, n = 1). All de-escalated patients and all who continued triple ART had suppressed HIV viremia at last follow-up and were satisfied with their ART regimen, except for one patient who had virological failure after ART discontinuation in the setting of major depression. The most common reasons to not de-escalate included hepatitis B co-infection (n = 6), physician's concern about ART adherence (n = 6), patient reluctance to switch from a single tablet to a 2-tablet regimen (n = 7), patient satisfied with current ART (n = 5) and others (n = 12).
Interpretation: ART de-escalation to dolutegravir/FTC or dolutegravir/3TC is possible in the majority of patients virologically suppressed on triple ART, and may effectively address patient and physician concerns about long-term safety and cost of ART.
Keywords: De-escalation; Dolutegravir; Dual antiretroviral therapy; HIV infection; Induction and maintenance treatment.
Figures
References
-
- Kooij K.W., Vogt L., Wit F.W.N.M. Higher prevalence and faster progression of chronic kidney disease in human immunodeficiency virus-infected middle-aged individuals compared with human immunodeficiency virus-uninfected controls. J Infect Dis. 2017;216:622–631. - PubMed
-
- Bedimo R., Maalouf N.M., Zhang S., Drechsler H., Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS. 2012;26:825–831. - PubMed
-
- Cahn P., Andrade-Villanueva J., Arribas J.R. Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect Dis. 2014;14:572–580. - PubMed
-
- Arribas J.R., Girard P.-M., Landman R. Dual treatment with lopinavir-ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a randomised, open-label, non-inferiority trial. Lancet Infect Dis. 2015;15:785–792. - PubMed
LinkOut - more resources
Full Text Sources

