Computational data of phytoconstituents from Hibiscus rosa-sinensis on various anti-obesity targets
- PMID: 31193691
- PMCID: PMC6538924
- DOI: 10.1016/j.dib.2019.103994
Computational data of phytoconstituents from Hibiscus rosa-sinensis on various anti-obesity targets
Abstract
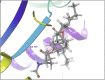
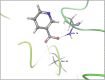
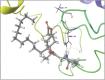
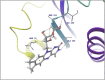
Molecular docking analysis of twenty two phytoconstituents from Hibiscus rosa-sinensis, against seven targets of obesity like pancreatic lipase, fat and obesity protein (FTO protein), cannabinoid receptor, hormones as ghrelin, leptin and protein as SCH1 and MCH1 is detailed in this data article. Chemical structures of phytoconstituents were downloaded from PubChem and protein structures were retrieved from RCSB protein databank. Docking was performed using FlexX software Lead IT version 2.3.2; Bio Solved IT. Visualization and analysis was done by Schrodinger maestro software. The docking score and interactions with important amino acids were analyzed and compared with marketed drug, orlistat. The findings suggest exploitation of best ligands experimentally to develop novel anti-obesity agent.
Figures
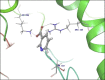
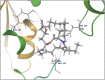

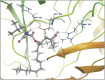

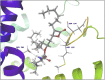



Similar articles
-
Attenuation of high glucose induced apoptotic and inflammatory signaling pathways in RIN-m5F pancreatic β cell lines by Hibiscus rosa sinensis L. petals and its phytoconstituents.J Ethnopharmacol. 2018 Dec 5;227:8-17. doi: 10.1016/j.jep.2018.08.022. Epub 2018 Aug 16. J Ethnopharmacol. 2018. PMID: 30120944
-
Identification and isolation of antimicrobial compounds from the flower extract of Hibiscus rosa-sinensis L: In silico and in vitro approaches.Microb Pathog. 2018 Oct;123:527-535. doi: 10.1016/j.micpath.2018.08.003. Epub 2018 Aug 4. Microb Pathog. 2018. PMID: 30086346
-
AMPK activating and anti adipogenic potential of Hibiscus rosa sinensis flower in 3T3-L1 cells.J Ethnopharmacol. 2019 Apr 6;233:123-130. doi: 10.1016/j.jep.2018.12.039. Epub 2018 Dec 26. J Ethnopharmacol. 2019. PMID: 30593890
-
Phenotypic Similarities in Flower Characteristics Between Novel Winter-Hardy Hibiscus Hybrids and Their Tropical Relatives.Front Plant Sci. 2019 Nov 22;10:1528. doi: 10.3389/fpls.2019.01528. eCollection 2019. Front Plant Sci. 2019. PMID: 31824544 Free PMC article. Review.
-
Do the Natural Chemical Compounds Interact with the Same Targets of Current Pharmacotherapy for Weight Management?-A Review.Curr Drug Targets. 2019;20(4):399-411. doi: 10.2174/1389450119666180830125958. Curr Drug Targets. 2019. PMID: 30173643 Review.
Cited by
-
Integrating network pharmacology analysis and pharmacodynamic evaluation for exploring the active components and molecular mechanism of moutan seed coat extract to improve cognitive impairment.Front Pharmacol. 2022 Aug 12;13:952876. doi: 10.3389/fphar.2022.952876. eCollection 2022. Front Pharmacol. 2022. PMID: 36034803 Free PMC article.
-
Preparative HPLC fraction of Hibiscus rosa-sinensis essential oil against biofilm forming Klebsiella pneumoniae.Saudi J Biol Sci. 2020 Oct;27(10):2853-2862. doi: 10.1016/j.sjbs.2020.07.008. Epub 2020 Jul 10. Saudi J Biol Sci. 2020. PMID: 32994746 Free PMC article.
-
Genomic landscape of the emerging XDR Salmonella Typhi for mining druggable targets clpP, hisH, folP and gpmI and screening of novel TCM inhibitors, molecular docking and simulation analyses.BMC Microbiol. 2023 Jan 21;23(1):25. doi: 10.1186/s12866-023-02756-6. BMC Microbiol. 2023. PMID: 36681806 Free PMC article.
-
In Vitro Antiprotozoal Activity of Hibiscus sabdariffa Extract against a Ciliate Causing High Mortalities in Turbot Aquaculture.Biology (Basel). 2023 Jun 26;12(7):912. doi: 10.3390/biology12070912. Biology (Basel). 2023. PMID: 37508344 Free PMC article.
References
-
- Min K.H., Yoo J., Park H. Computer-aided identification of ligands for GPCR anti-obesity targets. Curr. Top. Med. Chem. 2009;9:539–553. - PubMed
-
- Sankar V., Engels S.M. Synthesis, biological evaluation, molecular docking and in silico ADME studies of phenacyl esters of N-phthaloyl amino acids as pancreatic lipase inhibitors. FJPS. 2018;4:276–283.
-
- Luigi B., Giacomo M., Valentina V., Renato P., Uberto P. Cannabinoid receptors as therapeutic targets for obesity and metabolic diseases. Curr. Opin. Pharmacol. 2006;6:586–591. - PubMed
-
- Tutone M., Lauria A., Almerico A.M. Leptin and the ob-receptor as anti-obesity target: recent in silico advances in the comprehension of the protein-protein interaction and rational drug design of antiobesity lead compounds. Curr. Pharmaceut. Des. 2014;20:001–010. - PubMed
LinkOut - more resources
Full Text Sources

