A phase 2 trial of N-Acetylcysteine in Biliary atresia after Kasai portoenterostomy
- PMID: 31193715
- PMCID: PMC6542754
- DOI: 10.1016/j.conctc.2019.100370
A phase 2 trial of N-Acetylcysteine in Biliary atresia after Kasai portoenterostomy
Abstract
Background: Biliary atresia (BA) is a life-threatening liver disease of infancy, characterized by extrahepatic biliary obstruction, bile retention, and progressive liver injury. The Kasai portoenterostomy (KP) is BA's only nontransplant treatment. Its success is variable and depends on restoration of hepatic bile flow. Many adjunctive therapeutics have been studied to improve outcomes after the KP, but none demonstrate effectiveness. This study tests if N-acetylcysteine (NAC), a precursor to the choleretic glutathione, improves bile flow after KP.
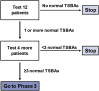
Methods: This report describes the design of an open-label, single center, Phase 2 study to determine the effect of NAC following KP on markers of bile flow and outcomes in BA. The intervention is intravenous NAC (150 mg/kg/day) administered continuously for seven days starting 0-24 h after KP. The primary outcome is normalization of total serum bile acid (TSBA) concentrations within 24 weeks of KP. The secondary objectives are to describe NAC therapy's effect on other clinical parameters followed in BA for 24 months and to report adverse events occurring with therapy. This study follows the "minimax" clinical trial design.
Discussion: This is the first clinical trial to test NAC's effectiveness in improving bile flow after KP in BA. It introduces three important concepts for future BA therapeutic trials: (1) the "minimax" study design, a pertinent design for rare diseases because it detects potential effects quickly with small subject size; (2) the more sensitive bile flow marker, TSBAs, which may correlate with positive long-term outcomes better than traditional bile flow markers such as serum bilirubin; and (3) liver enzyme changes immediately after KP, which can be a guideline for potential drug-induced liver injury in other BA peri-operative adjunctive therapeutic trials.
Keywords: ALT, Alanine transaminase; AST, Aspartate aminotransferase; BA, Biliary atresia; Bc, Conjugated bilirubin; Biliary atresia; DILI, Drug-induced liver injury; DSMB, Data and Safety Monitoring Board; DoL, Day of life; Drug-induced liver injury; FDA, Food and Drug administration; GGT, Gamma-glutamlytransferase; IOC, Intraoperative cholangiogram; KP, Kasai portoenterostomy; Kasai portoenterostomy; Minimax design; N-acetylcysteine; NAC, N-acetylcysteine; START, Steroids in Biliary Atresia Randomized Trial; Serum bile acids; TB, Total bilirubin; TCH, Texas Children's Hospital; TSBA, Total serum bile acids.
Figures

Similar articles
-
A phase 2 trial of short-term intravenous N-acetylcysteine in biliary atresia after Kasai portoenterostomy.Hepatol Commun. 2025 Jun 9;9(7):e0729. doi: 10.1097/HC9.0000000000000729. eCollection 2025 Jul 1. Hepatol Commun. 2025. PMID: 40489761 Free PMC article. Clinical Trial.
-
Five-year native liver survival analysis in biliary atresia from a single large Chinese center: The death/liver transplantation hazard change and the importance of rapid early clearance of jaundice.J Pediatr Surg. 2019 Aug;54(8):1680-1685. doi: 10.1016/j.jpedsurg.2018.09.025. Epub 2018 Oct 30. J Pediatr Surg. 2019. PMID: 30518490
-
The Fecal Microbiome in Infants With Biliary Atresia Associates With Bile Flow After Kasai Portoenterostomy.J Pediatr Gastroenterol Nutr. 2020 Jun;70(6):789-795. doi: 10.1097/MPG.0000000000002686. J Pediatr Gastroenterol Nutr. 2020. PMID: 32443032
-
High-dose steroids, ursodeoxycholic acid, and chronic intravenous antibiotics improve bile flow after Kasai procedure in infants with biliary atresia.J Pediatr Surg. 2003 Mar;38(3):406-11. doi: 10.1053/jpsu.2003.50069. J Pediatr Surg. 2003. PMID: 12632357 Review.
-
Biliary atresia: pathogenesis and treatment.Semin Liver Dis. 1998;18(3):281-93. doi: 10.1055/s-2007-1007164. Semin Liver Dis. 1998. PMID: 9773428 Review.
Cited by
-
Diseases of bile duct in children.World J Gastroenterol. 2024 Mar 7;30(9):1043-1072. doi: 10.3748/wjg.v30.i9.1043. World J Gastroenterol. 2024. PMID: 38577180 Free PMC article. Review.
-
Impact of Parenteral Lipid Emulsion Components on Cholestatic Liver Disease in Neonates.Nutrients. 2021 Feb 4;13(2):508. doi: 10.3390/nu13020508. Nutrients. 2021. PMID: 33557154 Free PMC article. Review.
-
Recent advances in the management of pediatric cholestatic liver diseases.J Pediatr Gastroenterol Nutr. 2025 Apr;80(4):549-558. doi: 10.1002/jpn3.12462. Epub 2025 Jan 22. J Pediatr Gastroenterol Nutr. 2025. PMID: 39840645 Review.
-
In Utero Extrahepatic Bile Duct Damage and Repair: Implications for Biliary Atresia.Pediatr Dev Pathol. 2024 Jul-Aug;27(4):291-310. doi: 10.1177/10935266241247479. Epub 2024 May 19. Pediatr Dev Pathol. 2024. PMID: 38762769 Free PMC article. Review.
-
Comparative analysis of cystic biliary atresia and choledochal cysts.Front Pediatr. 2022 Aug 24;10:947876. doi: 10.3389/fped.2022.947876. eCollection 2022. Front Pediatr. 2022. PMID: 36090570 Free PMC article.
References
-
- Jimenez-Rivera C., Jolin-Dahel K.S., Fortinsky K.J., Gozdyra P., Benchimol E.I. International incidence and outcomes of biliary atresia. J. Pediatr. Gastroenterol. Nutr. 2013;56:344–354. - PubMed
-
- Shneider B.L., Brown M.B., Haber B., Whitington P.F., Schwarz K., Squires R., Bezerra J., Shepherd R., Rosenthal P., Hoofnagle J.H., Sokol R.J. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J. Pediatr. 2006;148:467–474. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

