Neutrophil-mimicking therapeutic nanoparticles for targeted chemotherapy of pancreatic carcinoma
- PMID: 31193785
- PMCID: PMC6543032
- DOI: 10.1016/j.apsb.2018.12.009
Neutrophil-mimicking therapeutic nanoparticles for targeted chemotherapy of pancreatic carcinoma
Abstract
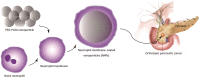
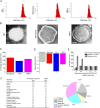
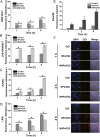
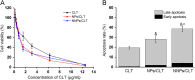
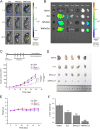
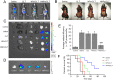
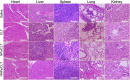
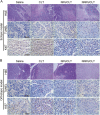
Due to the critical correlation between inflammation and carcinogenesis, a therapeutic candidate with anti-inflammatory activity may find application in cancer therapy. Here, we report the therapeutic efficacy of celastrol as a promising candidate compound for treatment of pancreatic carcinoma via naïve neutrophil membrane-coated poly(ethylene glycol) methyl ether-block-poly(lactic-co-glycolic acid) (PEG-PLGA) nanoparticles. Neutrophil membrane-coated nanoparticles (NNPs) are well demonstrated to overcome the blood pancreas barrier to achieve pancreas-specific drug delivery in vivo. Using tumor-bearing mice xenograft model, NNPs showed selective accumulations at the tumor site following systemic administration as compared to nanoparticles without neutrophil membrane coating. In both orthotopic and ectopic tumor models, celastrol-loaded NNPs demonstrated greatly enhanced tumor inhibition which significantly prolonged the survival of tumor bearing mice and minimizing liver metastases. Overall, these results suggest that celastrol-loaded NNPs represent a viable and effective treatment option for pancreatic carcinoma.
Keywords: 5-FU, fluorouracil; CLT, celastrol; Celastrol; DAPI, 4′,6-diamidino-2-phenylindole; DiD, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate; IKKα, IκB kinase α; IKKβ, IκB kinase β; IL-1β, interleukin 1 beta; IL-6, interleukin 6; Inflammation; NF-κB, nuclear factor kappa B; NIK, NF kappa B inducing kinase; NNPs, neutrophil membrane-coated nanoparticles; NPs, nanoparticles without neutrophil membrane coating; Naïve neutrophils membrane; PEG-PLGA nanoparticle; PEG-PLGA, poly(ethylene glycol) methyl ether-block-poly(lactic-co-glycolic acid); PI, propidium iodide; Pancreatic carcinoma; TAK1, TGF-β-activated kinase 1; TEM, transmission electronic microscopy; TNF-α, tumor necrosis factor alpha.
Figures


References
-
- Ryan D.P., Hong T.S., Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. - PubMed
-
- Kamisawa T., Wood L.D., Itoi T., Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. - PubMed
-
- Zhang S.H., Zhang H., He H.W., Li Y., Li X.Y., Zhang L.F. In vivo real-time imaging of gemcitabine-leaded growth inhibition in the orthotopic transplantation model of human pancreatic tumor. Acta Pharm Sin B. 2011;1:220–225.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

