CXCR4 in human osteosarcoma malignant progression. The response of osteosarcoma cell lines to the fully human CXCR4 antibody MDX1338
- PMID: 31193811
- PMCID: PMC6543022
- DOI: 10.1016/j.jbo.2019.100239
CXCR4 in human osteosarcoma malignant progression. The response of osteosarcoma cell lines to the fully human CXCR4 antibody MDX1338
Abstract
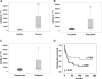
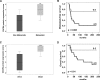
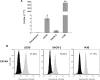
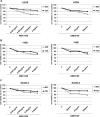
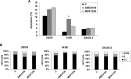
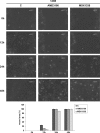
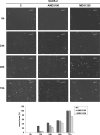
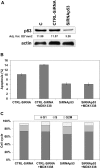
Osteosarcoma (OS) is the most frequent primary malignant tumour of bone and metastases occur in 30% of cases, the 5-year survival rate is 25-30%. Although pre- and post-operative chemotherapy has improved prognosis in osteosarcoma (OS), high toxicity and natural and acquired drug-resistance are the first cause of treatment failure. The identification of new predictive and therapeutic biomarkers may increase drug sensitivity and better control localized and metastatic disease. By the evidence that CXCR4 receptor by binding its ligand CXCL12 activates downstream critical endpoints for tumour malignancy, we first studied human OS progression correlating CXCR4 expression in OS biopsy with patient clinical data. By Real-time PCR and immunoistochemistry we found that high levels of CXCR4 gene and protein expression significantly correlated with OS progression, emphasizing the role of CXCR4/CXCL12 axis in tumour prognosis. This was supported by univariate analyses that showed a higher probability of local and/or systemic relapse in OS patients with a high CXCR4 gene expression and a significant increase of metastasis risk associated with an increasing score of CXCR4 protein staining intensity. Secondarily, to study the role of CXCR4 as a target for new therapeutic strategies, we evaluated the response of OS cells to the fully human CXCR4 antibody, MDX1338. In the study we also included AMD3100, the most studied CXCR4 antagonist. In CXCR4-positive OS cells cultured in CXCL12-rich BM-MCS-CM (bone marrow-derived mesenchymal stem conditioned medium), a decrease of cell proliferation up to 30%-40% of control was seen after drug exposure. However, an increase of apoptosis was seen in p53-positive U2OS and 143B after CXCR4 inhibitor incubation, while no changes were seen in treated SAOS-2 cells which also present a different labeling profile. The role of p53 in apoptotic response to CXCR4 inhibitors was confirmed by p53 silencing in U2OS cell line. Our data suggest that the response to anti-CXCR4 agents could be influenced by the genetic background and labeling profile which induces a different cross-talk between tumour cells and environment. The delay in cell cycle progression associated with increased apoptosis could sensitize p53-positive cells to conventional therapy and in vivo preclinical experiments are on going with the aim to suggest new combined target therapies in human OS.
Keywords: Biomarkers; CXCR4 antagonists; Metastasis; Prognosis; Sarcoma.
Figures


Similar articles
-
CXCR4 antibody treatment suppresses metastatic spread to the lung of intratibial human osteosarcoma xenografts in mice.Clin Exp Metastasis. 2014 Mar;31(3):339-49. doi: 10.1007/s10585-013-9632-3. Epub 2014 Jan 4. Clin Exp Metastasis. 2014. PMID: 24390633 Free PMC article.
-
A novel antagonist of CXCR4 prevents bone marrow-derived mesenchymal stem cell-mediated osteosarcoma and hepatocellular carcinoma cell migration and invasion.Cancer Lett. 2016 Jan 1;370(1):100-7. doi: 10.1016/j.canlet.2015.10.018. Epub 2015 Oct 27. Cancer Lett. 2016. PMID: 26517945
-
The importance of the CXCL12-CXCR4 chemokine ligand-receptor interaction in prostate cancer metastasis.J Exp Ther Oncol. 2004 Dec;4(4):291-303. J Exp Ther Oncol. 2004. PMID: 15844659
-
The role of the CXCL12-CXCR4/CXCR7 axis in the progression and metastasis of bone sarcomas (Review).Int J Mol Med. 2013 Dec;32(6):1239-46. doi: 10.3892/ijmm.2013.1521. Epub 2013 Oct 11. Int J Mol Med. 2013. PMID: 24127013 Review.
-
Overview of resistance to systemic therapy in patients with breast cancer.Adv Exp Med Biol. 2007;608:1-22. doi: 10.1007/978-0-387-74039-3_1. Adv Exp Med Biol. 2007. PMID: 17993229 Review.
Cited by
-
Clinicopathological and prognostic significance of CXCR4 expression in osteosarcoma: a meta-analysis.Biomedicine (Taipei). 2022 Dec 1;12(4):34-43. doi: 10.37796/2211-8039.1360. eCollection 2022. Biomedicine (Taipei). 2022. PMID: 36816176 Free PMC article.
-
Spatial profiling identifies regionally distinct microenvironments and targetable immunosuppressive mechanisms in pediatric osteosarcoma pulmonary metastases.bioRxiv [Preprint]. 2025 Jan 24:2025.01.22.631350. doi: 10.1101/2025.01.22.631350. bioRxiv. 2025. Update in: Cancer Res. 2025 Jun 16;85(12):2320-2337. doi: 10.1158/0008-5472.CAN-24-3723. PMID: 39896512 Free PMC article. Updated. Preprint.
-
Spatial Profiling Identifies Regionally Distinct Microenvironments and Targetable Immunosuppressive Mechanisms in Pediatric Osteosarcoma Pulmonary Metastases.Cancer Res. 2025 Jun 16;85(12):2320-2337. doi: 10.1158/0008-5472.CAN-24-3723. Cancer Res. 2025. PMID: 40173049
-
Current Status of 68Ga-Pentixafor in Solid Tumours.Diagnostics (Basel). 2022 Sep 2;12(9):2135. doi: 10.3390/diagnostics12092135. Diagnostics (Basel). 2022. PMID: 36140541 Free PMC article. Review.
-
Cluster of differentiation 133 (CD133) and C-X-C chemokine receptor 4 (CXCR4) associated with the incidence of metastasis in osteosarcoma patients.Eur J Orthop Surg Traumatol. 2025 Jul 23;35(1):318. doi: 10.1007/s00590-025-04434-x. Eur J Orthop Surg Traumatol. 2025. PMID: 40699365 Free PMC article.
References
-
- Picci P. Classic osteosarcoma. In: Manfrini M., Fabbri N., Gambarotti M., Vanel D., editors. Atlas of Muscoloskeletal Tumors and Tumorlike Lesions. Springer International Publishing; 2014. pp. 147–151.
-
- Klein M.J., Siegal G.P. Osteosarcoma: anatomic and histologic variants. Am. J. Clin. Pathol. 2006;4:555–581. - PubMed
-
- Harrison D.J., Schwartz C.I. Osteogenic sarcoma: systemic chemotherapy options for localized disease. Curr. Treat. Options Oncol. 2017;18:24. - PubMed
-
- Osanan S., Zang M., Shen F., Paul P.J., Persad S., Sergi C. Osteogenic Sarcoma: a 21st century review. Anticancer Res. 2016;36:4391–4398. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

